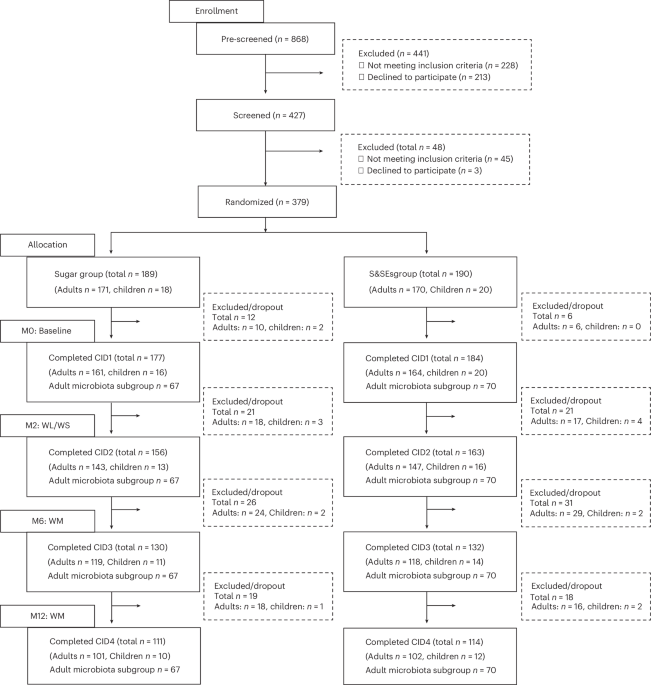
Ethics statement This trial has been registered at ClinicalTrials.gov (NCT04226911), was approved by national ethical committees (protocols and amendments) and was conducted in accordance with the Declaration of Helsinki. The study was monitored for Good Clinical Practice compliance by the European Clinical Research Infrastructure Network, as described previously18. All participants provided written informed consent. Trial design The SWEET trial was …
Read More »