For Immediate Release: September 19, 2025 Today, the U.S. Food and Drug Administration granted accelerated approval to Forzinity (elamipretide) injection as the first treatment for Barth syndrome, in patients weighing at least 30 kg. Barth syndrome is a rare, serious and life-threatening disease of the mitochondria (the energy-producing parts of cells). “The FDA remains committed to facilitating the development of …
Read More »Tag Archives: Barth
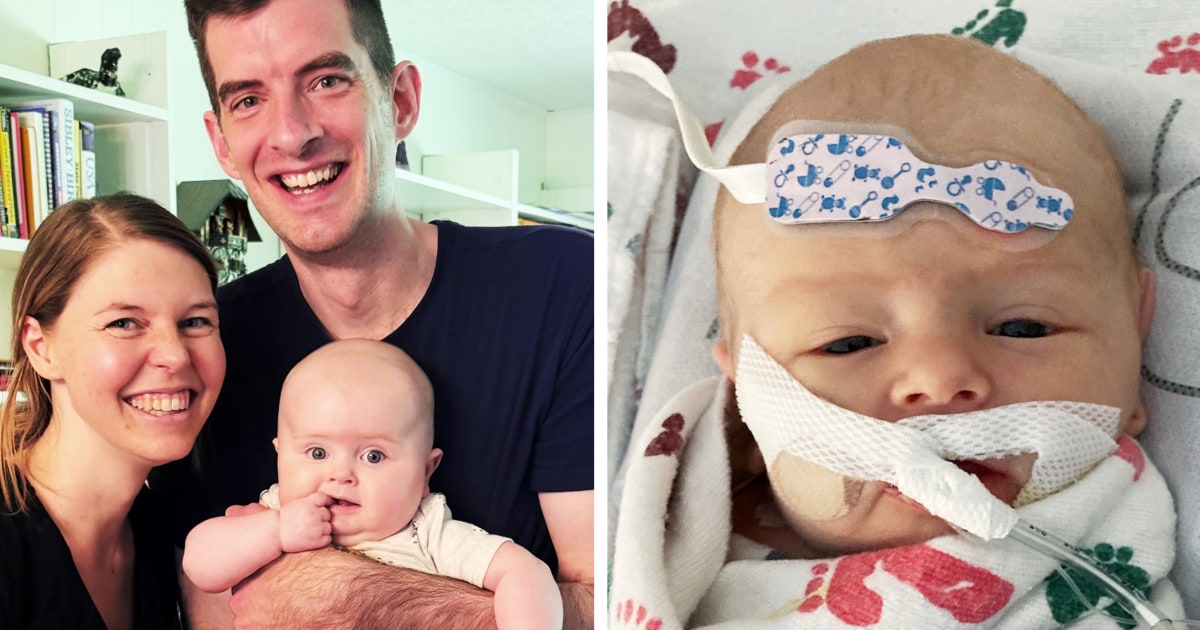
For kids with Barth syndrome, time is running out, parents say, unless the FDA acts
Gilbert Dryden probably only has enough medication to get him through the end of October, his mother, Madison, figures. Seven-month-old Gilbert has a rare genetic condition called Barth syndrome, one that can have dire consequences, like heart failure, extreme muscle weakness and a dramatically reduced life expectancy. Children who die early often don’t see their fifth birthday. Two infant deaths …
Read More »