Sometimes big breakthroughs in science come from very small changes, curiosity, and more than a little luck. A new study from researchers at Oregon Health & Science University shows how swapping a single atom in a known compound created an experimental cancer-fighting molecule with a completely different mode of action and improved properties.
Although drug discovery takes plenty of smarts, without a little luck, you probably won’t get very far. A recent study led by Yali Zhang and colleagues at the Oregon Health & Science University Knight Cancer Institute and published in Cell Reports Medicine offers a good example: a tiny atomic change to a familiar natural product yielded a structurally similar experimental molecule with very different properties. It has the potential to fight cancer by a completely different mechanism.
Breast cancer takes many forms, defined largely by the receptors that sit on the surface of tumor cells. The most common are hormone and growth factor receptors for estrogen, progesterone, and HER2. The pattern of these receptors determines how the cancer behaves and which treatments work best. For example, cancers driven by estrogen, progesterone, or HER2 can be treated with targeted drugs, but tumors missing all three receptors—known as triple-negative breast cancers—are much harder to treat and have been the focus of intense research.
Some of these aggressive cancers are linked to inherited BRCA mutations, which also limit treatment options. These cancers often rely on altered energy use to survive, depending heavily on glycolysis, a fast way of producing energy even when oxygen is available.
The Zhang group set out to refine podophyllotoxin, a natural product long known for its anticancer activity but also for its toxicity. The compound is derived from the roots of the mayapple plant, a traditional medicinal source that also gave rise to several important chemotherapy drugs such as etoposide and teniposide. Podophyllotoxin works by binding directly to the active (this is called orthosteric) site on β-tubulin, the same region targeted by colchicine (an autumn crocus alkaloid that’s been used forever for gout and was later found to bind tubulin and block microtubule assembly).
The researchers aimed to make the molecule safer and more effective, and more water soluble through subtle atomic changes—a concept known as bioisosterism, in which one atom or group is replaced by another to fine-tune a molecule’s behavior. In this case, they introduced a nitrogen atom into the ring system and replaced the oxygen-rich methylenedioxy group with a cyclopentane ring (Figure 1).
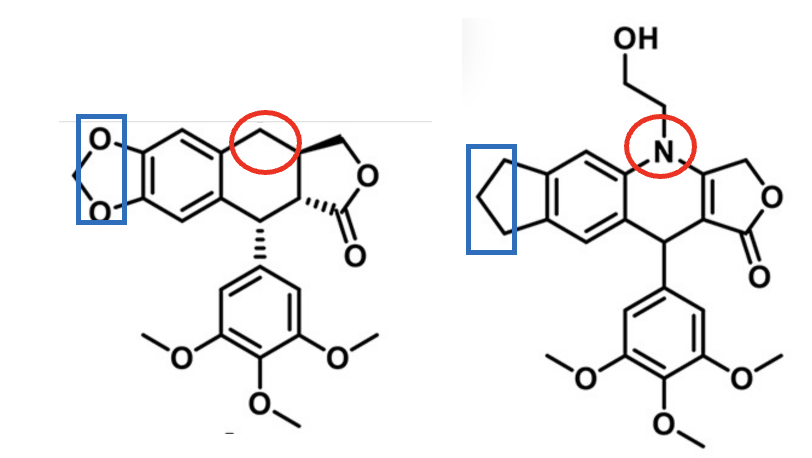
Figure 1. (Left) Podophyllotoxin, a toxic, poorly soluble tubulin inhibitor. (Right) Aza-podophyllotoxin (SU212), a less toxic bioisostere that no longer binds tubulin. Blue boxes and red circles highlight the structural changes between the two molecules.
These small adjustments were intended to improve stability and reduce side effects, but they also produced a surprise. The modified molecule, also called SU212, no longer acted on tubulin at all. Instead, it is bound to a different protein involved in cancer metabolism—enolase 1 (ENO1). Rather than blocking cell division, SU212 disrupted the tumor’s energy supply. Acting at a non-orthosteric site on ENO1, it caused the enzyme to break down, which slowed the growth and spread of triple-negative, HER2-negative breast cancers in experimental models with minimal toxicity in cultured cells and mice. [2]
This unexpected finding is hardly unprecedented in drug discovery. The creation of carbamorphine, a nitrogen-substituted version of morphine that I have written about, resulted in a compound that retained analgesic properties while lowering addiction potential. In much the same way, SU212’s design turned a known cytotoxin into a metabolic inhibitor, illustrating how progress in medicinal chemistry often arises from a mix of careful design and fortunate discovery, aka brains and luck.
Now, much more luck will be required. Going from an experimental compound to an approved drug is a long and dangerous journey.
NOTES:
[1] Sometimes the properties of a molecule can be improved by a single-atom change for reasons other than direct binding. For example, atoms that don’t affect how the molecule interacts with its target may be modified to improve solubility, stability, or ease of synthesis. It’s the same concept. [2] In cell and animal models, SU212 inhibited glycolysis and energy production in triple-negative breast cancer cells, decreasing ATP and lactate levels and promoting proteasomal degradation of ENO1, with little effect on normal cells. These details are beyond the scope of this article.Source link


