Scientists have created the first sizable meteorite diamond — also known as lonsdaleite or hexagonal diamond — a material predicted to be even harder than the diamonds normally found on Earth.
The high-pressure, high-temperature technique created tiny disks of this ultrahard diamond which could ultimately replace conventional diamonds in applications such as drilling tools and electronics, the scientists reported July 30 in the journal Nature.
Diamonds hold the record for the world’s hardest naturally occurring substance. Each carbon atom in the infinitely repeating molecular structure forms four equal-length bonds to other carbon atoms, each separated by a 109.5 degree angle, to create an endless array of perfect tetrahedra. Viewed from the side, this structure appears to contain three repeating layers of carbon atoms (labeled A, B, and C), and this gives diamond what crystallographers call a face-centered cubic crystal structure.
In the 1960s, however, a subtly different structure of diamond was proposed, with small impure crystals of this structure subsequently discovered in the Canyon Diablo meteorite, which crashed in the Arizona desert around 50,000 years ago.
Unlike in cubic diamond, this form contains two different bond lengths — one slightly longer than in normal diamond and one slightly shorter. The carbon atoms are still organized into endless planes of tetrahedra. But this time, when viewed from the side, the structure contains only two repeating layers (labeled A and B). This slight shift in the carbon layers gives meteorite diamond a hexagonal structure, which scientists theorize should boost the solid’s hardness by 58%.
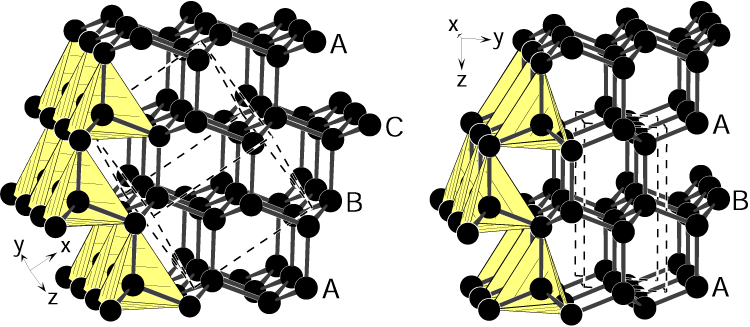
But preparing samples of this hexagonal structure large enough to analyze has been challenging. What’s more, the presence of other contaminating forms of carbon in the original meteorite sample — including graphite, cubic diamond and amorphous carbon — led many to doubt whether hexagonal diamond exists at all.
Related: Why do diamonds come in different colors?
Inspired by the Canyon Diablo meteoric fragment, Wenge Yang and colleagues at the Center for High Pressure Science and Technology Advanced Research in Beijing, sought to reproduce the intense conditions of an impact with Earth in the lab, developing a high-pressure, high-temperature synthesis using a diamond anvil cell, a piece of equipment that squashes a sample between two flattened surfaces made of diamond. Starting from another form of carbon, purified graphite, they slowly and carefully compressed the material, fixing the shifted atoms in place with targeted heat from a laser.

“At pressures around 20 GPa (200,000 atmospheres), the flat carbon layers of graphite are forced to slide and bond with adjacent layers, forming a buckled carbon honeycomb characteristic of hexagonal diamond,” Yang told Live Science in an email. “Laser heating above 1400 °C [2,552 Fahrenheit] facilitates this transition.” Once these distorted tetrahedra of hexagonal diamond had formed, the team slowly released the pressure, ensuring the new crystal didn’t spontaneously turn back into graphite.
The team then used powerful techniques to view the crystal structure and confirm their achievement. Although the crystal disk remained somewhat impure, containing random fragments of cubic diamond, electron microscope images clearly showed its AB carbon layers, and X-ray crystallography revealed the hexagonal structure.
“It’s a good first demonstration,” said Soumen Mandal, a physicist who specializes in the applications of diamond at the University of Cardiff in the U.K., who was not involved in the study. “Now we need pure crystals and more material to start exploring its physical and mechanical properties, thermal properties, electric properties, all of these.”
Hardness testing generally requires larger samples than the ones Yang’s team produced, according to the study. However, they did confirm the new material was at least as tough as regular diamondsl and Yang hopes subsequent experiments with larger and purer crystals will soon provide a concrete answer.
The team would ultimately like to see hexagonal diamond begin to replace conventional diamond in industrial technologies such as precision machinery, high-performance electronics, quantum technologies and thermal management systems, although such applications may still be 10 years away.
“Looking forward, our goal is to produce larger, high-quality hexagonal diamond samples suitable for real-world applications,” he said. “These efforts will help tailor hexagonal diamond’s properties for specific applications and pave the way for its industrial adoption.”
Source link

