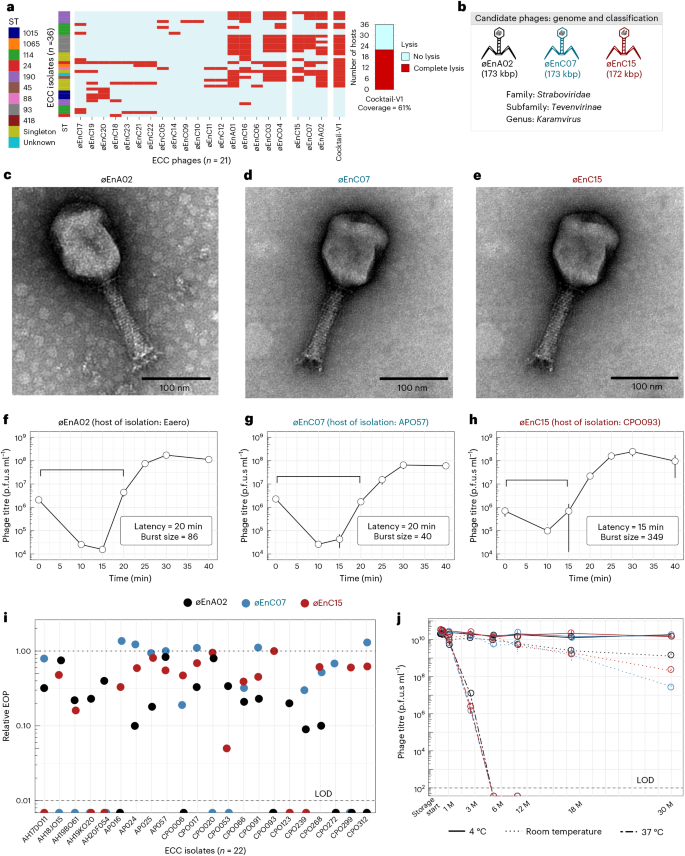
Isolation and characterization of phages against Enterobacter isolates from The Alfred Hospital
The Alfred Hospital in Melbourne, Australia, has reported an ongoing outbreak of nosocomial infections with limited treatment options and high mortality rates29. Of particular concern is the ECC, which is an emerging AMR threat with considerable epidemic potential22. Here we explored phages as a frontline antimicrobial solution for The Alfred Hospital’s ECC outbreak. Using clinical ECC isolates collected over a 10-year period, we selected a subset of 36 ECC isolates that represent the sequence type (ST) diversity of the full isolate collection (Supplementary Data 1). We then constructed an initial phage library against these isolates. After isolating and purifying 21 phages, we screened their plaque-forming capacity against our isolate subset to obtain a host-range map (Fig. 1a). Of the 36 isolates, 34 were susceptible to at least one phage. However, considering the time and cost associated with characterizing and producing each therapeutic phage, we aimed to build a phage combination that offered the broadest host range with the fewest possible phage combinations. On the basis of the complementary broad-spectrum host range, we selected øEnA02, øEnC07 and øEnC15 to produce the initial ECC phage combination (cocktail-V1), which provided 61% coverage against the 36 sub-selected ECC hosts (Fig. 1a). It should be noted that these phages were selected on the basis of host-range coverage, and their phage receptor targets were unknown at the time. All phages in the cocktail were sequenced and their genomes were analysed to ensure that they did not carry genes known to allow lysogeny or virulence, including integrases, recombinases, mobile genetic elements or genes encoding antibiotic resistance or toxicity. On the basis of genome similarity, we classified the phages at the genus level (Fig. 1b, and Supplementary Figs. 1 and 2). Electron microscopy imaging (Fig. 1c–e) revealed icosahedral capsids and sheathed contractile tails with lateral tail fibres, consistent with the Tevenvirinae subfamily within the Straboviridae family30. Importantly, therapeutically relevant bacteriophages must be capable of efficient replication and effectively suppress the growth of target strains. To this end, we evaluated the replicative characteristics of the three phages on their host of isolation using the one-step growth curve, revealing that øEnA02 (host: Eaero) had a burst size of 86 per infected cell with a latency of 20 min (Fig. 1f), øEnC07 (host: APO57) had a burst size of 40 with a latency of 20 min (Fig. 1g), and øEnC15 (host: CPO093) had a burst size of 349 with a latency of 15 min (Fig. 1h). Next, we verified the killing efficacy of the 3-phage cocktail as assessed by efficiency of plating (EOP) to evaluate how well the cocktail phages could infect other permissive hosts31. The results demonstrated at least one phage within the cocktail with an EOP between 0.1 and >1 for each host except for two instances: øEnA02 against CPO239, and øEnC15 against CPO053 (Fig. 1i).
a, Host-range map of isolated phages (columns, n = 21) and cocktail-V1 tested against a subset of 36 ECC isolates. Each row represents an ECC isolate, classified on the basis of ST and colour coded accordingly. Singletons are STs that only occurred once. b, Representations of phage genome size and classification of the three phages (øEnA02, øEnC07 and øEnC15) of cocktail-V1. c–e, TEM images of the three phages (øEnA02 (c), øEnC07 (d) and øEnC15 (e)). f–h, One-step growth curve of the three phages (øEnA02 (f), øEnC07 (g) and øEnC15 (h)) in the cocktail propagated on their host of isolation. Data represent mean ± s.e.m of 3 biological replicates. The latency time (in minutes) and burst size (in p.f.u.s per infection) were calculated for each phage. i, Relative EOP values for three phages, represented by different coloured dots (n = 1) across ECC isolates. The upper dotted line represents the EOPs on the host of isolation, which are set as 1. The lower dashed line represents the limit of detection (LOD) of the assay. j, Stability of the three phages (øEnA02, øEnC07 and øEnC15) at 4 °C, room temperature and 37 °C storage conditions. Data represent mean ± s.e.m of 2 biological replicates. M, months.
With our goal to produce a frontline antimicrobial preparation, phage stability under variable storage conditions is paramount32,33. We examined the stability of each phage within the cocktail. Cleaned phage lysates in LB media were stored individually in polypropylene tubes under storage conditions at 4 °C, room temperature (~22 °C) and 37 °C for 30 months, with phage titrations performed at different intervals. Within the initial 6 months, no noticeable drop in titre occurred in lysates stored at 4 °C and room temperature, yet over the following 30 months, 4 °C proved the most stable storage condition. Phages stored at 37 °C were the least stable, losing therapeutic titre within 2 months (Fig. 1j). Collectively, these data suggest that these three phages have broad host range and high antimicrobial efficacy against ECC isolates, and demonstrate excellent stability, making them promising candidates for further development and evaluation.
Identification of phage receptors
Bacteria can quickly evolve resistance against phage predation, which typically manifests via loss-of-function mutations in the surface-associated structures that phages adsorb to. The simultaneous use of diverse phages targeting different bacterial surface structures has been reported to minimize or delay the evolution of phage resistance34,35. To identify the receptors involved in phage–host interaction, we generated phage-resistant mutants which were sequenced to examine loss-of-function mutations, focusing on lipopolysaccharide (LPS) and other cell surface related genes36. We identified putative phage receptors by mapping the raw sequencing reads of phage-resistant mutants to the wild-type (WT) host genome. To validate these phage receptors, we complemented the candidate wild-type gene back to its respective phage-resistant mutant and performed an adsorption assay to determine whether phage infectivity was restored. We found that each of the three phages recognized a different component of the LPS structure, which was used to mediate adsorption and subsequent infection (Fig. 2a). For the phage-resistant mutant øEnA02, we identified two loss-of-function mutations: the first in an O-antigen-associated gene (wcaJ) (Fig. 2b)37 and the second in an outer membrane protein (ompW) (Fig. 2c), with both mutations resulting in the gain of an early stop codon. O-antigen is a serogroup-specific sugar-based component of the outer LPS layer of Gram-negative bacteria and is a well-characterized phage receptor38,39,40. OmpW has been shown to be a phage receptor for Vibrio cholerae phage41,42,43, having important roles in virulence and acting as a colicin receptor in E. coli, suggesting potential fitness trade-offs associated with phage resistance42,43. Following complementation, we quantified øEnA02 adsorption to the WT host, each of the two phage-resistant mutants and each mutant complemented with the respective WT gene (Fig. 2b,c). The WT host adsorbed ~1 log of phage within 10 min, while both phage-resistant mutants showed minimal adsorption. Comparatively, the complemented mutant restored phage adsorption, albeit only partially for the OmpW mutant (Fig. 2c), suggesting that øEnA02 uses OmpW as a secondary receptor. The øEnC07-resistant mutant had a loss-of-function mutation in a glycosyltransferase gene (Fig. 2d), which is part of the rfa operon and associated with LPS core synthesis44,45. Complementing the mutant with the WT gene restored phage infectivity and increased phage adsorption by 0.2 log. Finally, øEnC15-resistant mutant had a stop codon in the UTP-glucose-1-phosphate uridylyltransferase gene (galU) (Fig. 2e), which encodes an enzyme for UDP-glucose synthesis, which in turn is a precursor for O-antigen synthesis46. Complementing galU into the phage-resistant mutant restored phage adsorption, indicated by a 0.6 log increase.
a, Schematic representation of the Enterobacter LPS and membrane structure, with validated phage receptors indicated by arrows. Colours are for illustrative purposes only and the faded section represents the potential loss of surface-associated structure in phage-resistant mutants. Figure drawn on the basis of ref. 96 using Inkscape. b, SNPs identified in the genome of øEnA02-resistant mutant (ø-R-mutant) and predicted effect on wcaJ. The graph on the right shows phage adsorption assay testing the ability of phages to adsorb to its corresponding WT, phage-resistant mutant and complemented host over 10 min. c, SNPs identified in the genome of øEnA02-resistant mutant and predicted effect on ompW with phage adsorption assay as shown in the graph on the right. d, SNPs identified in the genome of øEnC07-resistant mutant and predicted effect on GT2 with phage adsorption assays as shown in the graph on the right. e, SNPs identified in the genome of øEnC15-resistant mutant and predicted effect on galU with phage adsorption assay as shown in the graph on the right. WT indicates wild-type host, ø-R-mutant indicates phage-resistant mutant, and numbers represent nucleotide positions for the respective genes. Data represent mean ± s.e.m of 3 biological replicates.
The identification of phage receptors provides mechanistic insights into phage infectivity and the emergence of resistance. While all our phages broadly targeted the LPS, this was mediated through recognition of distinct LPS subunits, which may reduce the emergence of phage resistance when used in combination (Supplementary Fig. 3a). We noted that phage activity was not fully restored in phage-resistant mutants after complementation, which may have been due to altered gene expression post complementation. In addition, phage-resistant mutants harboured several single nucleotide polymorphisms (SNPs) in non-target genes with either known or hypothetical functions (Supplementary Data 2), and we cannot exclude the possibility that these mutations contributed to partial restoration or altered adsorption. This remains an area for future investigation.
In vivo effectiveness of the preliminary phage cocktail-V1
After establishing the host range, infectivity, genomes, stability and receptors for our preliminary 3-phage combination (cocktail-V1), we evaluated its efficacy in reducing ECC burden in vivo using a murine model. For this model, we selected ECC isolate APO57, which was susceptible to all three phages at an EOP of ~1. We optimized the ECC inoculum dose to induce severe septicaemia in 8-week-old BALB/c mice, reaching an ethical endpoint within 12 h (Supplementary Fig. 3b,c). Mice were intraperitoneally injected with 200 µl of an optimized dose of 5 × 106 colony-forming units (c.f.u.s) ml−1 of isolate APO57. At 1 h post infection (hpi), mice were treated with a single dose of cocktail-V1, which contained 108 plaque-forming units (p.f.u.s) ml−1 of each of the three phages in 200 µl, while control mice received an equivalent volume of sterile PBS. The experiment concluded at 12 hpi and vital organs were collected to assess the bacterial and phage load (Extended Data Fig. 1a). The phage-cocktail treatment resulted in >3 log reduction (>99.9%) in the bacterial burden in the infected mouse’s blood, kidney, liver and spleen compared with the PBS control group (p < 0.05) (Extended Data Fig. 1b). Notably, bacterial load in the blood was cleared below our limit of detection in four out of six mice. Phage load assessment revealed propagation of all three phages in vivo, indicating successful replication and dissemination throughout the mice (Extended Data Fig. 1c). We further observed that the liver and spleen exhibited higher phage concentrations compared with the blood and kidney (p < 0.05). However, there was no significant difference in phage concentrations when comparing the blood to the kidney or the liver to the spleen. Our phage cocktail development was an iterative process, and this in vivo experiment served as a key checkpoint to assess efficacy. Due to its capacity to reduce bacterial burden and ability to replicate effectively in mouse organs, we advanced the 3-phage combination to the next stage of clinical evaluation.
Efficacy of cocktail-V1 against the wider collection of clinical ECC isolates
At this stage, we sought to determine the effectiveness of our phage cocktail-V1 against the broader clinical collection of ECC isolates from The Alfred Hospital. We conducted high-throughput screening of the lytic activity of cocktail-V1 and its individual components via a spot assay against 120 clinical ECC isolates (Extended Data Fig. 1d). At the time, this represented the entire ECC collection at The Alfred Hospital, encompassing a range of STs that contributed to the nosocomial outbreak. Two researchers who were blinded to the experiment visually scored the zones on the bacterial lawn as complete lysis, partial lysis, or no lysis (Supplementary Fig. 3d). The 3-phage cocktail lysed 54.2% of the 120 ECC isolates, which was a decrease of ~7% in lysis when compared against the initial 36-isolate collection cocktail-V1 was built upon (Fig. 1a). Instances of partial lysis were difficult to interpret and may represent low efficiency infections, emerging phage resistance, or bacterial lysis resulting from high phage doses without active propagation (killing from without)47. We observed an association between phage susceptibility and ST of the ECC isolates, with STs 114, 190 and 1015 being the least susceptible to the cocktail. The modest host-range coverage of 54.2%, along with limited activity against specific STs underscored the need for a more tailored approach to phage isolation and expansion of phage infectivity to ensure comprehensive coverage across broader STs encompassing the ECC nosocomial outbreak.
Phage adaptation to improve infectivity
A fundamental difference between phages and antibiotics is that phages can evolve and adapt to changes in their hosts. This can be harnessed to improve the fitness and antimicrobial efficacy of select phages48,49,50. Using an experimental evolution approach, we set out to improve the lytic capacity of the three phages from cocktail-V1. Our first goal was to determine the optimum phage adaptation (that is, training) duration that would result in improved lytic capacity. For this, we selected ECC isolate AH17D011 and øEnC07, a phage–host pair with an EOP of 0.79 (Fig. 1i). This phage–host pair was propagated for 24 h, followed by purification of the population of adapted phages, which were used to infect the naive ECC host, with the entire process being repeated for 10 days. From day 5 onwards, we performed growth kinetics assays to compare bacterial growth in the presence and absence of adapted phages to evaluate changes in their lytic efficacy, which are reported as phage scores (Extended Data Fig. 2)51,52. Phage scores take growth kinetics data and integrate these into a single value from 0 to 1 (Methods), with higher values representing greater phage fitness and infectivity. We observed that the phage score increased sharply from day 5 of adaptation and plateaued afterwards. On the basis of these observations, we selected 7 days as a sufficient phage adaptation period for further experiments. Importantly, following phage adaptation, evolved phages were twice plaque purified to ensure that a single phage genotype was taken for downstream characterization, including lytic activity via EOPs, growth curve and genomic changes (Fig. 3a).
a, Experimental protocol for phage adaptation. Ancestral phage was propagated with target hosts for 24 h. Subsequently, the purified phage population was repropagated on a naive population of the same host, with this cycle repeated for 10 days. After 5 days, daily measurements of phage growth curves were conducted using the evolved population to find the optimum adaptation duration. The final evolved phages (that is, day 7) were isolated via two rounds of single-plaque purification to obtain an individual genotype of evolved phage mutants. Then, the growth characteristics of these single-genotype evolved phages were assessed. b–d, The relative EOPs of evolved phages (øEnA02 (b), øE2nC07 (c) and øEnC15 (d)) compared to their ancestral counterparts. The labels on top of the boxes represent the adaptation ECC host. Each point (n = 6) represents data from independent biological replicates. p values were calculated using a two-tailed t-test with Welch’s correction. Box bounds indicate 25th and 75th percentiles. e, Phage score of ancestor phages compared to their respective evolved phages, tested against a subset of 36 ECC isolates. Each dot represents a phage score (n = 36) and horizontal lines show means. p values were calculated using a two-tailed t-test with Welch’s correction. f–h, One-step growth curve of evolved phages (øEnA02 (f), øE2nC07 (g) and øEnC15 (h)) compared to their ancestral counterparts, propagated on their respective adaptation hosts. Data represent mean ± s.e.m of n = 3 biological replicates. Y axis represents phage titre, normalized to 100% at time 0. Data are displayed on a logarithmic scale (base 10) for visualization. The latency time (in minutes) and burst size (in p.f.u.s per infection) were calculated for each phage. Panel a created with BioRender.com.
We then examined whether our phage adaptation protocol could enhance lytic activity against hosts that had lower EOPs and were less responsive to a specific phage. As such, we adapted the remaining two phages from our cocktail: øEnA02 with host AH19K020 (EOP 0.2), and øEnC15 with a non-permissive host CPO390 (EOP below the limit of detection). Following phage adaptation, EOPs improved by 4,000-fold for øEnA02 (p < 0.001) and by 8.7-fold for øEnC07 (p = 0.033) compared with their ancestor (Fig. 3b,c). Intriguingly, øEnC15, which did not produce plaques or show lytic activity via growth assays with host CPO390, was found to infect and propagate at an EOP of 0.4 post adaptation (Fig. 3d). This unexpected finding suggests that phage adaptation may have overcome barriers, such as host defence mechanisms or surface structures that initially block infection53,54, warranting further investigation into these underlying factors.
To evaluate the broader fitness of adapted phages and any potential trade-offs, we performed growth kinetics assays to compare the lytic activity of ancestral phages to their evolved counterparts across our 36 ECC isolate subset, with data reported as phage score (Fig. 3e and Extended Data Fig. 3). The evolved phages showed an increased average phage score for øEnC07 (p = 0.078) and øEnC15 (p = 0.048), while øEnA02 showed minimal change (p = 0.84). To determine whether the observed improvements in the killing efficiency of the evolved phages were accompanied by changes in their life cycles, we conducted one-step kill curves on their adaptation hosts. Compared with its ancestor, the evolved øEnA02 had a faster replicative cycle as demonstrated by a shorter latency period (20 min vs 26 min) and released more progeny virions (burst size; 21 vs 15) (Fig. 3f). Evolved øEnC07 showed an enhanced adsorption rate, as indicated by the steeper decline of free phage percentage in the first 15 min of infection, with a comparable latency (22 min vs 20 min) and larger burst size (19 vs 11) (Fig. 3g). As for the øEnC15, we lacked an ancestral phage that could infect the host for the comparison, so we evaluated the lifecycle parameters of the evolved generation, revealing a latent period of ~18 min and a burst size of 10 (Fig. 3h).
To explore the molecular mechanisms underlying the improved phage efficacy, we conducted genome sequencing and comparative analysis between the evolved phages and their ancestors (Extended Data Fig. 4). We identified several SNPs in the evolved phages, with the majority located in the tail region of the genome, including the tail fibre and receptor-recognizing proteins. These proteins are critical for the initial adsorption of phages to host bacteria. Interestingly, we found that some of the SNPs were unique to certain phage–host pairs, indicating the specificity of the evolutionary process. On the basis of our observation, we hypothesized that the improved phage efficacy resulted from selection and accumulation of beneficial mutations during the adaptation process, which enabled the phages to better recognize and bind to their hosts. These phenotypic improvements and genotypic changes emerged within 7 days. However, this timeline and outcome may vary depending on the specific phage–host combination and should not be generalized. Collectively, these results demonstrate that our phage adaptation approach successfully improved phage infectivity against inefficient or even non-permissive hosts, via mutations in phage tail structures, impacting divergent variables of the phage lifecycle and enhancing killing efficiency.
Targeted phage isolation against problematic STs
Thus far, we focused on characterizing three phages of our cocktail-V1 and demonstrated their in vitro and in vivo efficacy, followed by phage adaptation to improve their lytic capacity. However, this 3-phage combination had limited effectiveness against specific STs from the ECC collection (Extended Data Fig. 1d). To expand coverage, we selected additional ECC isolates, including the problematic STs 190, 114 and 1015, which lacked sufficient phage coverage, as hosts for targeted phage isolation. Five additional phages were isolated and their activity tested via spot assay (Extended Data Fig. 5a). On the basis of the host-range map and complementary spectrum of activity provided by cocktail-V1, we selected two candidate phages (øNando and øTaquito) for further characterization (Fig. 4a). Genomic and taxonomic classification revealed that both phages belonged to the genus Pseudotevenvirus under the Straboviridae family (Fig. 4b). Transmission electron microscopy (TEM) revealed phages with an icosahedral head and a long contractile tail, consistent with the characteristics of the Straboviridae family (Fig. 4c,d). The genomes of both phages lack transposase or integrase genes, antimicrobial resistance markers and virulence genes, indicating that they are not temperate and are suitable candidates for therapeutic use.
a, Host-range map of isolated phages and cocktail-V1 tested against the initial subset of 36 ECC isolates. Each row represents a phage. STs are colour coded. Singletons are STs that occurred once. Red boxes show complete lysis and light blue boxes show no lysis. b, Representations of phage genome size and classification of the phages øNando and øTaquito. c,d, TEM images of the øNando (c) and øTaquito (d) phages. e, Schematic representation of the Enterobacter LPS and membrane structure, with putative phage receptors indicated by arrows. Colours are for illustrative purposes only and the faded section represents the potential loss of LPS structure in phage-resistant mutants. Figure drawn on the basis of ref. 96 using Inkscape. f,g, One-step growth curves of øNando (f) and øTaquito (g), propagated on their host of isolation. Data represent mean ± s.e.m. of 3 biological replicates. The latency time (in minutes) and burst size (in p.f.u.s per infection) were calculated for each phage. h, Stability tracking of øNando and øTaquito over 18 months (M), with storage at 4 °C and room temperature. Data represent mean ± s.e.m. of 2 biological replicates.
Identification of receptors revealed that both phages targeted different bacterial LPS surface structures to mediate infection (Fig. 4e). For the øNando-resistant host (mutated from isolate CPO448), we found a loss-of-function mutation within wzzB, which encodes a protein involved in the biosynthesis of O-antigen and helps determine the chain length55. In addition, for øTaquito-resistant host (isolation host CPO165), we discovered a loss-of-function mutation within the rfaQ gene. RfaQ is involved in LPS biosynthesis as it transfers the first two heptose residues in the inner core of LPS, and its loss leads to a severely truncated LPS alongside pleiotropic effects on bacterial cells44. We next evaluated one-step growth curves for both phages, with øNando (host: CPO448) demonstrating a latency of 15 min and a burst size of 88 (Fig. 4f) and øTaquito (host: CPO165) demonstrating a latency of 18 min and a burst size of 13 (Fig. 4g). Regarding stability, cleaned phage lysates stored in LB media at 4 °C maintained stability for up to 18 months (Fig. 4h), while room temperature storage supported sufficient phage stability for at least 6 months. These findings, combined with our initial phage results, warranted the inclusion of these two additional phages to our cocktail in an effort to expand host-range coverage against problematic STs that cocktail-V1 did not target.
Improved 5-phage cocktail achieved broad coverage against clinical ECC isolates
Our first-generation, 3-phage cocktail (cocktail-V1) achieved a modest ~54.2% host-range coverage against the ECC isolates endemic to The Alfred Hospital. To improve its coverage and efficacy, we adapted the original three phages to enhance their lytic activity against selected hosts, followed by a targeted isolation of two phages against problematic STs. Our focus then shifted to identifying the optimal combination of these phages for the development of an improved cocktail. To this end, we prepared eight combinations of phages, each containing either the three ancestral or three evolved phages, along with one or both of the two newly isolated phages (øNando and øTaquito), resulting in 3-, 4- or 5-phage formulations (Extended Data Fig. 5b). We tested these cocktails and a PBS control (9 samples labelled A-I) using a spot assay against an expanded collection of 156 clinical ECC isolates, which included our previous panel (n = 120) (Extended Data Fig. 1d) plus 36 additional ECC isolates that caused infections at The Alfred Hospital during the timeline of this study. Both of our 5-phage combinations, utilizing evolved phages (Combination G: ev_øEnA02, ev_øEnC07, ev_øEnC15, øNando and øTaquito) and ancestral phages (Combination I: øEnA02, øEnC07, øEnC15, øNando and øTaquito) produced complete lysis via spot assays against 65% and 75% of the collection, respectively. This was a marked improvement over cocktail-V1’s 43.9% spot lysis on the expanded collection, which included problematic and untargeted STs 190, 114 and 1015 (Fig. 5a and Extended Data Fig. 5b). Next, to resolve whether partial lysis results were indicative of productive infection, we used growth kinetics assays and calculated phage scores. We selected 67 hosts for analysis, including all hosts that produced partial lysis results, along with several hosts showing complete lysis as controls. To distinguish productive infections, an optimum cut-off value of 0.28 in phage score was determined using the maximum-likelihood estimation56. Most hosts with partial lysis zones on spot assays yielded productive infections, except for 12 hosts in Combination G and 9 in Combination I. All instances of complete lysis spots were confirmed to be productive, except for one host in Combination G. In addition, no difference in the phage scores was observed between Combinations G and I (Fig. 5b). Combining these results with the spot assay data, we deduced that both Combinations G and I achieved productive infection rates of at least 92% (144 out of 156 isolates) (Fig. 5c). Given our previous demonstration of enhanced fitness and lytic replication in the adapted phages compared with their ancestral counterparts (Fig. 3e), Combination G was selected for manufacturing stable, therapeutic-grade phage product.
a, Host-range coverage of phage combinations (G and I) tested against 156 ECC isolates on the basis of spot assay data. b, Comparison of phage scores of combinations G and I against ECC isolates. Each dot represents a phage score (n = 67). p values were calculated using a two-tailed t-test with Welch’s correction. Dashed line (<0.28) indicates the threshold for characterizing a phage activity as productive. c, Host-range coverage (n = 156) of phage combinations (G and I), based on combined phage score and spot assay data. d, Schematic representation of the two-stage therapeutic-grade phage production protocol developed by the Monash Phage Foundry. e, Phage titre at each production stage. Each data point represents results from three biological replicates. Each boxplot color represents a specific phage shown at the top of the graph. Whiskers indicate maximum and minimum values, individual points beyond whiskers are outliers, box bounds indicate 25th and 75th percentiles, and centre line indicates the median. f, Endotoxin activity as EU per ml of pure lysate [stage 2 (iii)] and the final product [stage 2 (vi)]. g, TEM images of phage product Entelli-02 showing intact virions and no contaminants. h, Normalized relative coverage (relative abundance) of sequencing reads from Entelli-02 against each component phage and predicted prophages from the clinical ECC production strains. i, Phage titre stability of the packaged Entelli-02 product with storage at 4 °C in 1× PBS supplemented with 1 mM CaCl2 over 18 months (M). Data represent mean ± s.e.m of 2 biological replicates. Panel d created with BioRender.com.
Production of a therapeutic-grade phage cocktail Entelli-02
We manufactured a therapeutic-grade phage product that we named ‘Entelli-02’ from the 5-phage combination (Combination G) at our in-house facility, the Monash Phage Foundry (Fig. 5d). Importantly, we define a therapeutic-grade phage product as having been produced under institutionally approved guidelines with endpoint quality control measures for sterility, endotoxins, phage activity and phage purity, and being suitable for intravenous administration to patients under Australia’s Therapeutic Goods Administration (TGA) Special Access Scheme (Category A). For the first stage of production, each phage was amplified individually through overnight propagation with their respective ECC hosts of isolation. The resulting phage lysates were processed using a two-stage sequential depth filtration (0.5–15 µm and 0.2–3.5 µm retention ratings) to reduce bacterial biomass, followed by sterilizing-grade (0.2 µm) filtration into sealed glass containers. Bacteria-free lysates (~1 l each) were then transferred to a clean-room facility for further processing. Phage lysates were diluted ~10-fold in 1× PBS, followed by buffer exchange and concentration using tangential flow filtration (TFF), resulting in a recovery of 130–160 ml of washed and concentrated lysate from each production run. We observed recovery efficiencies ranging from 24% to 76%, with a p.f.u.s ml−1 count exceeding 1010 p.f.u.s ml−1 for each phage (Fig. 5d and Extended Data Table 1).
For stage two of production, we mixed and, if necessary, diluted the five concentrated lysates to achieve a uniform phage product with a titre of >109 p.f.u.s ml−1 per phage in a total of 75 ml (Fig. 5e). We then depleted endotoxin using both EndotrapHD and 1-octanol treatments, resulting in nearly 10-fold reduction in endotoxin levels (Fig. 5f). Finally, we diluted the cocktail 10-fold in 1× PBS supplemented with 1 mM CaCl2 to obtain our final product consisting of an average titre of ~5 × 108 p.f.u.s ml−1 phage−1 in a total volume of 500 ml. The cocktail was twice filter sterilized using sterilizing-grade filters (0.2 µm), followed by quality control validation for phage titre and endotoxin levels (Methods). The cocktail was then packaged into syringe-accessible glass vials, each containing 35 ml of Entelli-02, which is suitable for a 2-week treatment course with an effective dose of 1 ml administered twice daily (b.i.d.). The final packed product underwent external validation for sterility and endotoxin, according to the USP71 and USP85 guidelines, respectively, with >10% of the production batch sent for validation. The final product contained no visible growth of microorganisms and had an endotoxin concentration of 1,575 endotoxin units (EU) ml−1, which according to FDA guidelines (5 EU × kg × h) would be safe for intravenous administration b.i.d. to a patient >30 kg (ref. 57). Electron microscopy images confirmed the visual integrity and cleanliness on Entelli-02 (Fig. 5g). Furthermore, considering that these phages were amplified on clinical isolates that contained potential prophages, we performed whole-genome sequencing of the final product followed by read mapping to the individual Entelli-02 phage genomes, the host bacterial genomes and predicted prophage regions. Sequence analysis showed that >99.2% of reads mapped to the Entelli-02 phage genomes, 0.27% to the bacterial genome, and ~0.09% to predicted prophage regions within the clinical production strains (Extended Data Table 2). To further examine the prophage presence, we determined the normalized coverage per million reads (relative abundance) of Entelli-02 phages and predicted prophages. Considering the average titre of ~5 × 108 p.f.u.s ml−1 phage−1 in our product and on the basis of the relative abundance of prophage genomes (~0.01%) (Fig. 5h), we inferred that the level of prophage contamination in Entelli-02 is <5 × 104 p.f.u.s ml−1. Finally, an important aspect of the chemistry, manufacturing and control process is maintaining the stability of the individual components over time. We measured titres of the individual phages using selective plating within our final Entelli-02 product and found no major loss of titre over 18 months of storage at 4 °C in 1× PBS supplemented with 1 mM CaCl2 (Fig. 5i), except for øEnA02, which showed ~1 log reduction.
Host range, phage resistance and antibiotic synergy of Entelli-02
We produced a therapeutic-grade Entelli-02 product, which demonstrated broad host coverage against ECC isolates from The Alfred Hospital. To evaluate its clinical relevance, we performed a final host-range screen of our Entelli-02 product against the entire ECC collection from The Alfred Hospital, which had increased to 206 clinical isolates during the timeline of this study. We conducted spot assays at three concentrations (107, 105 and 103 p.f.u.s ml−1) and assessed relative EOPs. We found that the majority of isolates (34%) were infected by a single phage within Entelli-02, ~25% of isolates were susceptible to two to three phages, 5% to four phages, and just 2% of isolates were susceptible to all five phages, while Entelli-02 failed to infect 12% of isolates (Fig. 6a). EOP heat maps indicated that most infections occurred between 0.1–1, with øEnA02 showing the lowest relative EOP of the five phages (Fig. 6b). Overall, Entelli-02 was able to infect 180 out of 206 ECC isolates at The Alfred Hospital.
a, Percentage host coverage of the 5 individual Entelli-02 phage components against 206 ECC isolates determined by plaque formation. b, EOP heat map for Entelli-02 phage components. Black bar indicates inferred overall host coverage (any detectable EOP) by Entelli-02. EOP scale divided at 0.001–0.1 and 0.1–1 for visual clarity. c, Comparison of phage scores following 5 days of serial passage of Entelli-02 on each host of isolation. Points represent mean phage scores (n = 6) from each 24-h kinetic growth measurements (OD600). d, Heat map comparing cross-resistance patterns of wild-type and 3 phage-resistant clones (R1–R3) against individual phages and Entelli-02. The right panel indicates the original phage and their host of isolation that was used for generation of resistance mutants. Dotted boxes highlight instances of complete cross-resistance (phage score <0.1). e, Interaction profiles between phages and antibiotics for wild-type and phage-resistant mutants (two clones; R1 and R2), tested against seven antibiotics from four different classes. Interactions are categorized as positive, negative or none on the basis of combined effects on bacterial growth. f,g, Bacterial burden in tissues of mice infected with ECC isolates APO57 (f) or AALF22D176 (g), comparing untreated controls versus cocktail-V1 or Entelli-02 treatment. Points represent individual mice (n = 6 per group). Boxplots show median (centre line), interquartile range (box edges: 25th–75th percentiles), the most extreme values within 1.5× interquartile range (whiskers), and outliers as individual points beyond whiskers. The total represents all samples combined (n = 24). Statistical analysis was performed using Mann–Whitney U-test (two-sided) with exact p values to compare medians of bacterial count between groups. The dashed line indicates the LOD. Right: phage propagation in tissues of treated mice. Each point represents phage titres from individual tissue samples (n = 24 total) of 6 mice, displayed as boxplots with medians and interquartile ranges as above. p values shown for phages with significant propagation; complete statistical comparisons in Extended Data Fig. 8c,f.
Next, we investigated emergence of phage resistance against Entelli-02. To mimic clinical use, we conducted a 5-day in vitro evolution experiment using the five hosts of isolation serially passaged with daily doses of Entelli-02. We measured phage scores of each host with Entelli-02, with lower scores correlating to reduced infectivity against the cocktail, probably due to the emergence of phage resistance (Fig. 6c and Extended Data Fig. 6a). By day 1, four of the hosts had phage scores between 0.7 and 0.95 suggesting that they were still susceptible to the Entelli-02 cocktail, with minimal emergence of phage resistance. Comparatively, host Eaero had a phage score of 0.33 by day 1, reflecting its low-level infectivity by phages in Entelli-02, compared with host APO57, which was highly susceptible to all five phages (Fig. 6d). Over the following 5 days, phage scores remained above 0.6 in four isolates, suggesting limited emergence of phage resistance with Entelli-02 (Fig. 6c).
To further investigate phage resistance impacts on bacterial infectivity and antibiotic interactions, we repeated our phage-resistance evolution experiments to isolate three new and independent phage-resistant mutants, for each component phage from Entelli-02, using their respective hosts of isolation (total of 15 mutants). These phage-resistant mutants were then screened for phage infectivity, which was assessed via phage score against each individual phage and the full Entelli-02 cocktail (Fig. 6d and Extended Data Fig. 6b). Broadly comparing the activity of Entelli-02 across the wild-type and phage-resistant mutants, our data suggest that when multiple phages exhibit strong lytic activity, Entelli-02 maintains efficacy despite the emergence of phage resistance. However, in cases where only one phage dominates the lytic activity (for example, øEnA02, øEnC15 or øTaquito), cross-resistance can reduce Entelli-02 effectiveness. This suggests that relying on one dominant phage increases the risk that resistance to it may compromise the entire cocktail. Other interesting observations include mutants resistant to øEnC07, whose APO57 host was initially sensitive to all five phages, lost sensitivity to øEnC15, øNando and øTaquito, but retained sensitivity to øEnA02. In contrast, øTaquito-resistant mutants were the least sensitive overall. Since øTaquito’s isolation host (CPO165) had low sensitivity to other Entelli-02 phages and its resistant mutants exhibited inner core LPS loss (Fig. 4e), this probably impaired receptor availability for subsequent phage infections. Interestingly, øEnA02 showed the highest activity against other phage-resistant mutants probably due to its dual receptors (Fig. 2b,c). These findings highlight the importance of designing phage cocktails with multiple active agents per host and where possible, including phages with diverse receptors to maximize therapeutic robustness and minimize cross-resistance. The use of receptor-diverse phages may offer additional benefits, such as reduced bacterial pathogenesis58 or increased sensitivity to certain antibiotic classes59. Finally, we screened whether our phage-resistant mutants incurred fitness costs60,61 through comparative growth curves, but did not observe any significant growth defects for phage-resistant mutants under standard growth in LB (Extended Data Fig. 6c).
Next, we evaluated the interaction between Entelli-02 and seven clinically relevant antibiotics (Fig. 6e), including β-lactams (meropenem, imipenem, cefepime, ceftazidime), an aminoglycoside (amikacin), a polymyxin (colistin) and a fluoroquinolone (ciprofloxacin) against the five isolation hosts and 10 phage-resistant mutants (selected from Fig. 6d) using growth kinetics assays. Each condition included: antibiotic alone (at 0.5× minimum inhibitory concentration (MIC)), Entelli-02 alone (at a multiplicity of infection (MOI) of 0.1), and combinations of both, totalling 315 interactions (Extended Data Fig. 7). Positive interactions indicate that the combination resulted in greater bacterial suppression than either treatment alone. No interaction meant that the combination performed similarly to the single treatments, while negative interactions indicated reduced suppression compared with the single treatments15. A limitation of the experiment was that when Entelli-02 achieved near-complete suppression (for example, APO57), it masked potential positive effects from combination treatments (Extended Data Fig. 7). Our analysis revealed that wild-type isolates broadly exhibited more frequent positive interactions with antibiotics than did the phage-resistant mutants. Notably, two wild-type isolates showed positive interactions with all antibiotics tested (Fig. 6e) In contrast, the phage-resistant mutants exhibited variable antibiotic interaction profiles, but among them, β-lactams demonstrated most positive interaction: 6 of 10 mutants interacted positively to ceftazidime, 4 to meropenem, and 3 each to imipenem and cefepime. This suggests that β-lactams could be promising partners for combination therapy with Entelli-02. In contrast, negative interactions with colistin were observed in 5/10 mutants. Colistin acts by electrostatically interacting with negatively charged LPS molecules, suggesting that our phage-resistant mutants, which have mutations in LPS-associated genes, probably impaired colistin’s efficacy62, further suggesting that colistin should be used cautiously in combination with phages, as has been previously described63,64. In addition, two negative interactions were observed with ciprofloxacin and phage-resistant mutants, while no interactions were found with amikacin despite positive effects seen in wild-type isolates, which may be associated with changes in cell permeability65. In summary, our Entelli-02 product broadly slowed the emergence of phage resistance and demonstrated positive interactions with clinically relevant antibiotics, particularly β-lactams, with some phage-resistant mutants exhibiting potential antibiotic resensitization events that could be exploited further clinically.
Preclinical evaluation of Entelli-02
After establishing the in vitro efficacy of Entelli-02 and characterizing its phage-resistance dynamics and antibiotic synergies, we proceeded to examine its efficacy in a murine infection model. We first replicated our previously established infection model with isolate APO57, which is sensitive to all five phages, by comparing the original 3-phage cocktail (cocktail-V1) with Entelli-02. Consistent with previous results, both cocktails reduced bacterial burden by ~3-logs in the blood, kidney, liver and spleen compared with the PBS control group (p < 0.05), with no differences between the two (p > 0.05) (Fig. 6f). Phage load analysis revealed that øEnA02 was the dominant phage in vivo, mirroring in vitro growth kinetics data where øEnA02 demonstrated the highest growth inhibition against APO57 (Extended Data Fig. 8a,b). Next, we used a contemporary clinical isolate AALF22D176 from The Alfred Hospital that was naïve to both cocktails, meaning none of the phages in cocktails were isolated or screened against it. This isolate represents a common sequence type (ST190) in the Alfred Hospital’s collection and was susceptible to all five phages in Entelli-02, although at different inhibition rates compared with APO57 (Extended Data Fig. 8d,e). While both cocktail-V1 and Entelli-02 suppressed bacterial load compared with the control (p < 0.05), Entelli-02 showed superior efficacy compared with cocktail-V1 (p < 0.05) (Fig. 6g). Phage replication also differed between the cocktails, with øEnC15 dominating in cocktail-V1-treated mice (p < 0.05), while øNando expanded the most in Entelli-02-treated mice (p < 0.05), which was consistent with in vitro growth kinetics (Extended Data Fig. 8d,e). These results demonstrate that the 5-phage Entelli-02 cocktail is comparable to the original 3-phage combination against host APO57 and offers improved bacterial suppression and phage propagation capacity in vivo against contemporary isolates that more accurately reflect clinical scenarios.
Source link