A genetically modified pig lung transplanted into a brain-dead human patient functioned for nine days in a new achievement that reveals both the promise and significant challenges of xenotransplantation.
Over the course of the experiment, the patient showed increasing signs of organ rejection before scientists at the First Affiliated Hospital of Guangzhou Medical University in China terminated the experiment, allowing the recipient to pass away.
It’s the first time a pig lung has been transplanted into a human patient, demonstrating a significant step forward, and giving scientists new problems to solve as they develop this emerging medical technique further.
Related: World-First Pig Kidney Transplant Was a Huge Breakthrough, But Is It The Future?
The availability of suitable human donor organs presents a major bottleneck for patients in need of a transplant. To help resolve this issue, doctors have been investigating the possibilities presented by xenotransplantation: genetically modifying organs from non-human animals – mainly pigs.
These modified pig organs are not intended to be permanent solutions for the patient, but a stop-gap ‘bridge‘ solution while they wait for a donor organ to become available. Clinical trials using pig kidneys and livers have shown promise, although further development and research is still needed.
Each organ presents its own unique complexities and obstacles. A team led by surgeon Jianxing He of Guangzhou Medical University has now tackled the next major challenge: lungs.
The goal of the experiment was not to achieve a successful transplantation on the first try – that would have been pretty incredible, but not a realistic expectation. Rather, the researchers wanted to observe how the patient’s immune system responded to the transplanted organ.
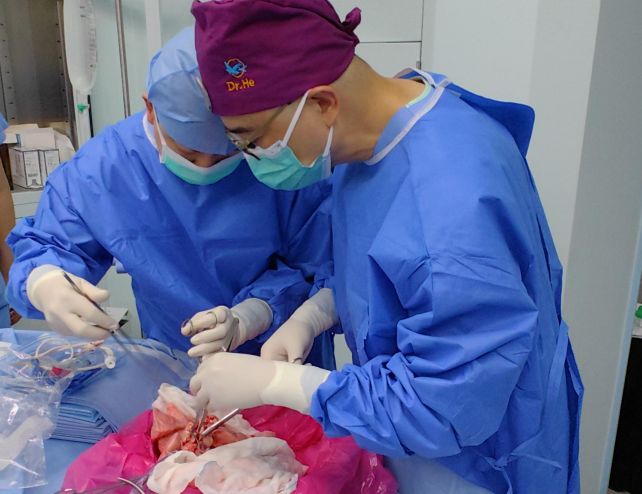
The patient was a 39-year-old man who was declared brain-dead by four separate clinical assessments after undergoing a brain hemorrhage. His family provided written informed consent for the experiment.
The donor pig is what is known as a six-gene-edited pig, a Bama miniature pig with six CRISPR gene edits, housed in an isolated facility with rigorous disinfection protocols. These edits are all focused on minimizing the immune and inflammatory responses of the patient.
In a careful surgical procedure, the pig’s left lung was placed into the patient’s chest cavity, and connected to their airways, arteries, and veins. The paper does not explain the fate of the pig, but donor pigs do not typically survive the removal of a major organ.
The patient was also treated with a number of immunosuppressants that the researchers adjusted according to changes observed in the patient’s body over time.
Initially, all seemed well, with none of the immediate signs of hyperacute rejection in the critical few hours following the procedure. However, by 24 hours after the transplant had taken place, severe swelling (edema) was observed, possibly as a result of blood flow being restored to the area of the transplant.
Antibody-mediated rejection damaged the tissue further on days three and six of the experiment. The result of the damage was primary graft dysfunction, a type of severe lung injury occurring within 72 hours of a transplant, and the leading cause of death in lung transplant patients. Some recovery was taking place by day nine, but the experiment had run its course.
Related: Pig Liver Successfully Transplanted Into Human Patient in World First
The lungs are a very complicated organ to transplant, because they have immediate contact with air from outside the body. This means they need to form an effective line of first defense, acting as a barrier against airborne pathogens and particles. As such, they have multiple mechanisms they can call on for an immune response.
The researchers were able to show that they could transplant a pig lung into a human patient in a way that circumvents the dangers of hyperacute rejection, which is an important first step.
“The early onset of pulmonary edema underscores the importance of preventing primary graft dysfunction in future xenogeneic lung transplantation,” the researchers write in their paper.
“Continued efforts are needed to optimize immunosuppressive regimens, refine genetic modifications, enhance lung preservation strategies and assess long-term graft function beyond the acute phase.
“By addressing these challenges, future studies can refine the approach to lung xenotransplantation and move closer to clinical translation. This study provides crucial insights into the immune, physiological and genetic barriers that must be overcome, and paves the way for further innovations in the field.”
The research has been published in Nature Medicine.
Source link


:max_bytes(150000):strip_icc()/kris-d55f6ce819fd4994a0f2651c0c299fd2.jpg)