Popular science background: They have created new rooms for chemistry (pdf)
Populärvetenskaplig information: De har skapat nya rum för kemi (pdf)

They have created new rooms for chemistry
Susumu Kitagawa, Richard Robson and Omar M. Yaghi are awarded the Nobel Prize in Chemistry 2025 for the development of a new type of molecular architecture. The constructions they created – metal-organic frameworks – contain large cavities in which molecules can flow in and out. Researchers have used them to harvest water from desert air, extract pollutants from water, capture carbon dioxide and store hydrogen.
An attractive and very spacious studio apartment, specifically designed for your life as a water molecule – this is how an estate agent might describe one of all the metal-organic frameworks that laboratories around the world have developed in recent decades. Other constructions of this type are tailormade for capturing carbon dioxide, separating PFAS from water, delivering pharmaceuticals in the body or managing extremely toxic gases. Some can trap the ethylene gas from fruit – so they ripen more slowly – or encapsulate enzymes that break down traces of antibiotics in the environment.

Simply stated, metal-organic frameworks are exceptionally useful. Susumu Kitagawa, Richard Robson and Omar Yaghi are awarded the Nobel Prize in Chemistry 2025 because they created the first metal-organic frameworks (MOF) and demonstrated their potential. Thanks to the laureates’ work, chemists have been able to design tens of thousands of different MOFs, facilitating new chemical wonders.
As so often in the sciences, the story of the Nobel Prize in Chemistry 2025 begins with someone who thought outside the box. This time, inspiration came during preparations for a classic chemistry lesson, in which the students were to build molecules from rods and balls.
A simple wooden model of a molecule generates an idea
It was 1974. Richard Robson, who was teaching at the University of Melbourne, Australia, had been tasked with turning wooden balls into models of atoms, so students could create molecular structures. For this to work, he needed the university’s workshop to drill holes in them, so that wooden rods – the chemical bonds – could be attached to the atoms. However, the holes could not be randomly placed. Each atom – such as carbon, nitrogen or chlorine – forms chemical bonds in a specific way. Robson needed to mark out where the holes should be drilled.
When the workshop returned the wooden balls, he tested building some molecules. This was when he had a moment of insight: there was a vast amount of information baked into the holes’ positioning. The model molecules automatically had the correct form and structure, because of where the holes were situated. This insight led to his next idea: what would happen if he utilised the atoms’ inherent properties to link together different types of molecules, rather than individual atoms? Could he design new types of molecular constructions?
Robson builds innovative chemical creations
Every year, when Robson brought out the wooden models to teach new students, the same idea occurred to him. However, more than a decade passed before he decided to test it out. He started with a very simple model, inspired by the structure of a diamond, in which each carbon atom bonds to four others, forming a tiny pyramid (figure 2). Robson’s aim was to build a similar structure, but his would be based on positively charged copper ions, Cu+. Like carbon, they prefer to have four other atoms around them.
He combined the copper ions with a molecule that has four arms: 4′,4″,4”’,4””-tetracyanotetraphenylmethane. There’s no need to remember its complicated name, but it is important that the molecule at the end of each arm had a chemical group, nitrile, that was attracted to the positively charged copper ions (figure 2).
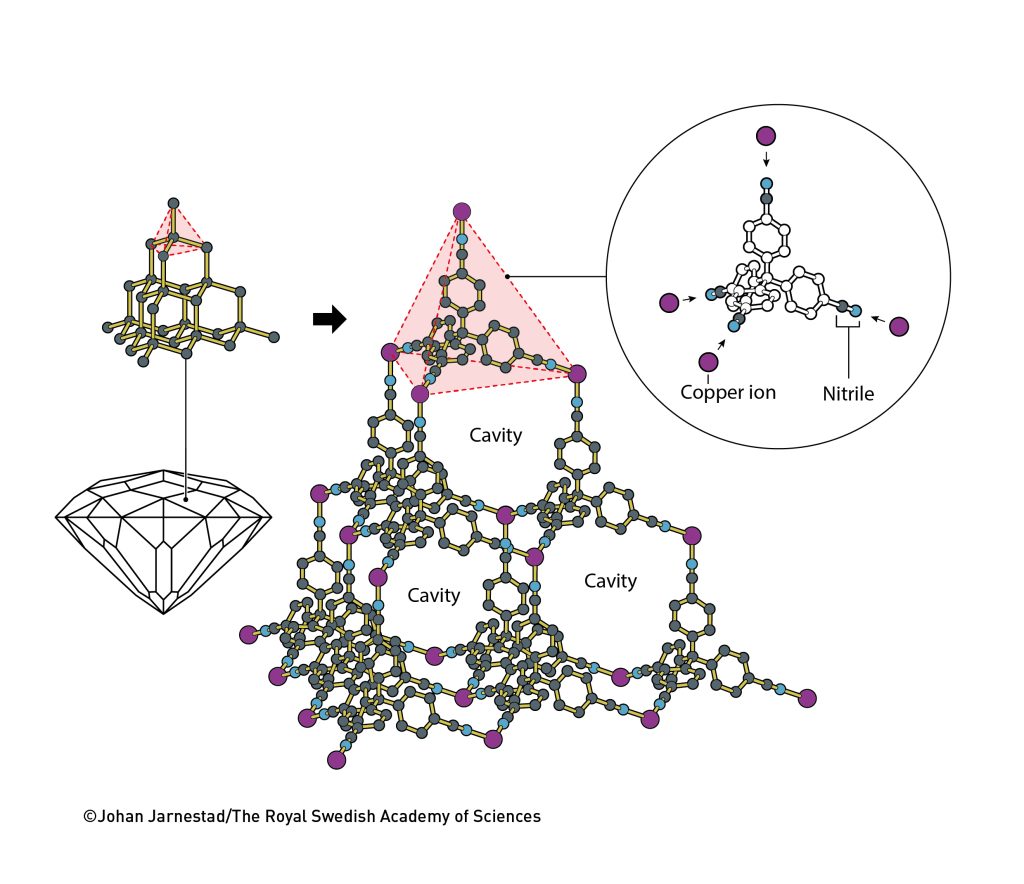
At that time, most chemists would have assumed that combining copper ions with the four-armed molecules would result in a bird’s nest of ions and molecules. But things went Robson’s way. As he had predicted, the ions and molecules inherent attraction to each other mattered, so they organised themselves into a large molecular construction. Just like carbon atoms in a diamond, they formed a regular crystalline structure. However, unlike diamond – which is a compact material – this crystal contained a vast number of large cavities (figure 2).
In 1989, Robson presented his innovative chemical creation in the Journal of the American Chemical Society. In his article, he speculates about the future and suggests that this could offer a new way to construct materials. These, he writes, could be given never previously seen properties, potentially beneficial ones.
As it turned out, he had foreseen the future.
Robson brings about a pioneering spirit in chemistry
As soon as the year after his pioneering work was published, Robson presented several new types of molecular constructions with cavities that were filled with various substances. He used one of them to exchange ions. He submerged the ion-filled construction in a fluid that contained a different type of ion. The result was that the ions changed places, demonstrating that substances could flow in and out of the construction.
In his experiments, Robson showed that rational design can be utilised for building crystals with spacious interiors that are optimised for specific chemicals. He suggested that this new form of molecular construction – when correctly designed – could be used to catalyse chemical reactions, for example.
However, Robson’s constructions were quite rickety and tended to fall apart. Many chemists thought they were useless, but some could see that he was onto something and, for them, his ideas about the future awakened a pioneering spirit. Those who would come to lay a stable foundation for his visions were Susumu Kitagawa and Omar Yaghi. Between 1992 and 2003 they made – separately – a series of groundbreaking discoveries. We will begin in the 1990s, with Kitagawa, who was working at Kindai University, Japan.
Kitagawa’s motto: even useless things can become useful
Throughout his research career, Susumu Kitagawa has followed an important principle: to try to see “the usefulness of useless.” As a young student, he read a book by the Nobel Prize laureate Hideki Yukawa. In it, Yukawa refers to an ancient Chinese philosopher, Zhuangzi, who says that we must question what we believe to be useful. Even if something does not bring immediate benefit, it may still turn out to be valuable.
Accordingly, when Kitagawa began to investigate the potential for creating porous molecular structures, he did not believe they had to have a specific purpose. When he presented his first molecular construction in 1992, it was indeed not particularly useful: a two-dimensional material with cavities in which acetone molecules could hide. However, it had resulted from a new way of thinking about the art of building with molecules. Like Robson, he used copper ions as cornerstones that were linked together by larger molecules.
Kitagawa wanted to continue experimenting with this new construction technology, but when he applied for grants, research funders did not think there was any particular point to his ambitions. The materials he created were unstable and had no purpose, so many of his proposals were rejected.
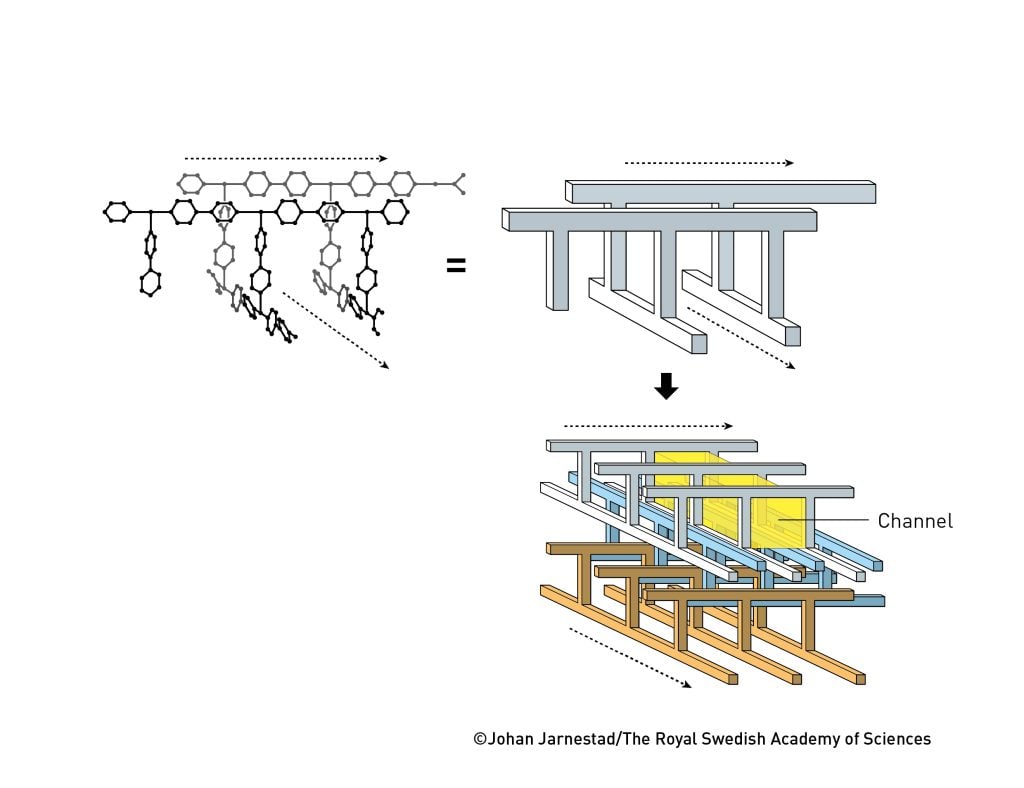
However, he did not give up and in 1997 he had his first major breakthrough. Using cobalt, nickel or zinc ions and a molecule called 4,4′-bipyridine, his research group created three-dimensional metal–organic frameworks that were intersected by open channels (figure 3). When they dried one of these materials – emptying it of water – it was stable and the spaces could even be filled with gases. The material could absorb and release methane, nitrogen and oxygen, without changing shape.
Kitagawa sees the uniqueness of his creations
Kitagawa’s constructions were both stable and had a function, but research funders were still unable to see their charm. One reason was that chemists already had zeolites, stable and porous materials, which they could build from silicon dioxide. These can absorb gases, so why would anyone develop a similar material that did not work as well?
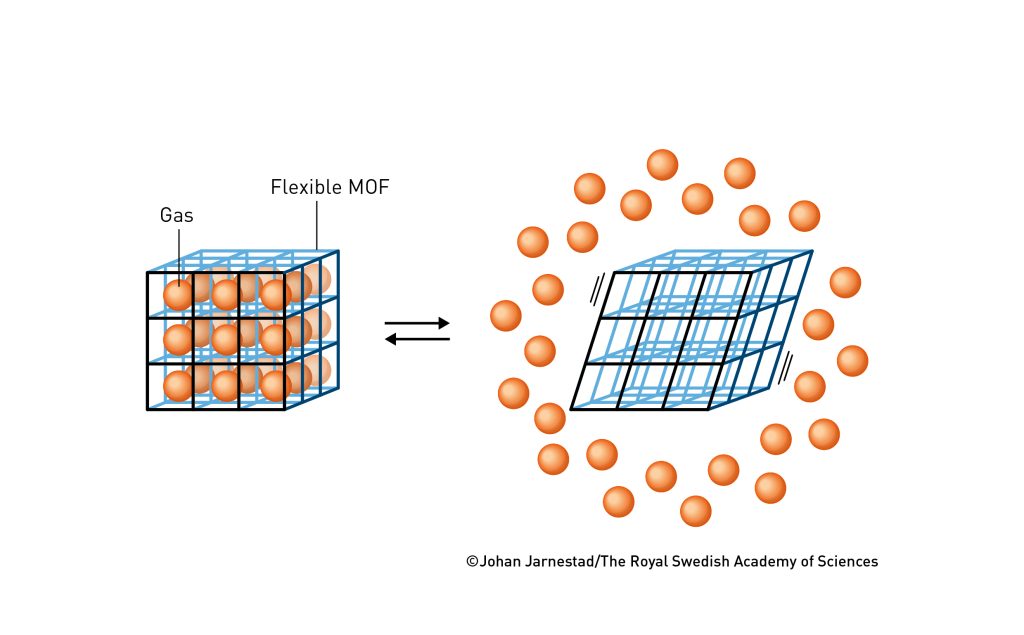
Susumu Kitagawa understood that if he were to receive any major grants, he had to define what made metal–organic frameworks unique. So, in 1998, he described his vision in the Bulletin of the Chemical Society of Japan. He presented several advantages with MOFs. For example, they can be created from many types of molecules, so there is enormous potential for integrating different functions. Also – and this is important – he realised that MOFs can form soft materials. Unlike zeolites, which are usually hard materials, MOFs contain flexible molecular building blocks (figure 4) that can create a pliant material.
After this, all he had to do was to put his ideas into practice. Kitagawa, along with other researchers, started developing flexible MOFs. While they work on this, we will move our focus to the US, where Omar Yaghi was also occupied with taking molecular architecture to new heights.
A secret library visit opens Yaghi’s eyes to chemistry
Studying chemistry was not an obvious choice for Omar Yaghi. He and his many siblings were raised in a single room in Amman, Jordan, with no electricity or running water. School was a refuge from his otherwise challenging life. One day, when he was ten years old, he sneaked into the school library, which was usually locked, and picked a book at random from the shelf. On opening it, his eyes were drawn to unintelligible but captivating pictures – his first encounter with molecular structures.
At the age of 15 – and on his father’s stern instruction – Yaghi moved to the US to study. He was attracted by chemistry and eventually by the art of designing new materials, but found the traditional way of building new molecules too unpredictable. Normally, chemists combine substances that are to react with each other in a container. Then, to start the chemical reaction, they heat the container. The desired molecule forms, but is also often accompanied by a range of contaminating side products.
In 1992, when Yaghi started his first position as research group leader, at Arizona State University, he wanted to find more controlled ways in which to create materials. His aim was to use rational design to connect different chemical constituents, like pieces of Lego, to make large crystals. This turned out to be challenging, but they finally succeeded when the research group started combining metal ions with organic molecules. In 1995, Yaghi published the structure of two different two-dimensional materials; these were like nets and were held together by copper or cobalt. The latter could host guest molecules in its spaces and, when these were fully occupied, it was so stable that it could be heated to 350°C without collapsing. Yaghi describes this material in an article in Nature where he coins the name “metal–organic framework;” this term is now used to describe extended and ordered molecular structures that potentially contain cavities, and are built from metals and organic (carbon-based) molecules.
Just a few grams of Yaghi’s framework can contain a football pitch
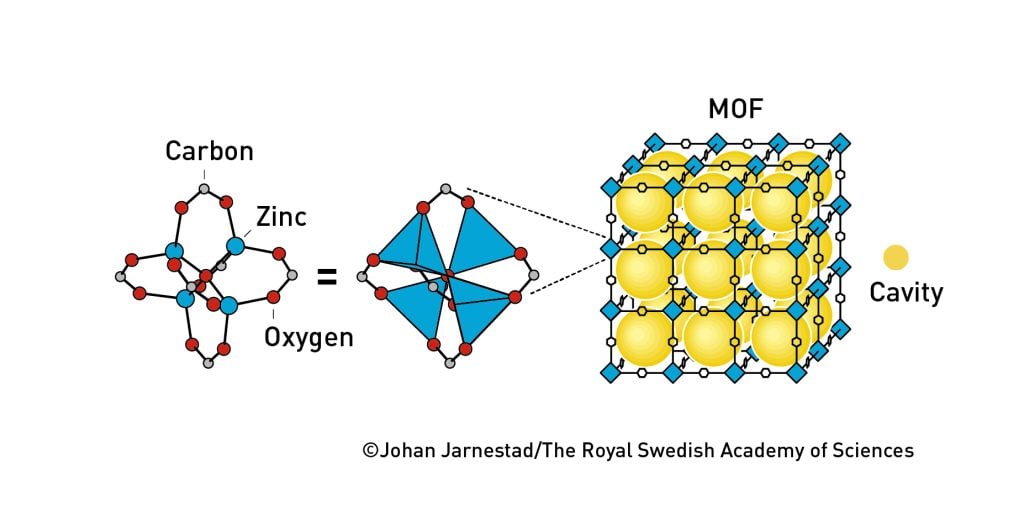
Yaghi established the next milestone in the development of metal–organic frameworks in 1999, when he presented MOF-5 to the world. This material has become a classic in the field. It is an exceptionally spacious and stable molecular construction. Even when empty, it can be heated to 300°C without collapsing.
However, what caused many researchers to raise their eyebrows was the enormous area hiding inside the material’s cubic spaces. A couple of grams of MOF-5 holds an area as big as a football pitch, which means it can absorb much more gas than a zeolite could (figure 5).
Speaking of the differences between zeolites and MOFs, it took just a few years for researchers to succeed in developing soft MOFs. One of those who was able to present a flexible material was Susumu Kitagawa himself. When his material was filled with water or methane, it changed shape, and when it was emptied, it returned to its original form. The material behaved somewhat like a lung that can breathe gas in and out, changeable but stable.
Yaghi’s research group conjures drinking water from desert air
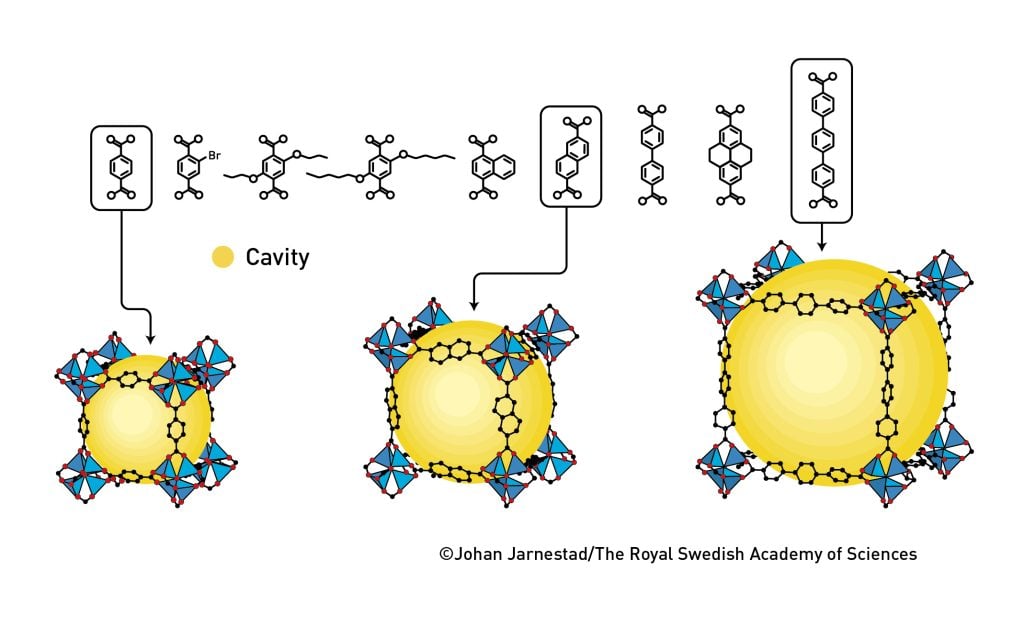
Omar Yaghi laid the final bricks in the foundation of metal–organic frameworks in 2002 and 2003. In two articles, in Science and Nature, he shows that it is possible to modify and change MOFs in a rational manner, giving them different properties. One thing he did was to produce 16 variants of MOF-5, with cavities that were both larger and smaller than those in the original material (figure 6). One variant could store huge volumes of methane gas, which Yaghi suggested could be used in RNG-fuelled vehicles.
Subsequently, metal–organic frameworks have taken the world by storm. Researchers have developed a molecular kit with a wide range of different pieces that can be used to create new MOFs. These have different shapes and characters, providing incredible potential for the rational – or AI-based – design of MOFs for different purposes. Figure 7 provides examples of how MOFs can be utilised. For instance, Yaghi’s research group has harvested water from the desert air of Arizona. During the night, their MOF material captured water vapour from the air. When dawn came and the sun heated the material, they were able to collect the water.
MOF materials that capture carbon dioxide and toxic gases
Researchers have created numerous different and functional MOFs. So far, in most cases, the materials have only been used on a small scale. To harness the benefits of MOF materials for humanity, many companies are now investing in their mass production and commercialisation. Some have succeeded. For example, the electronics industry can now use MOF materials to contain some of the toxic gases required to produce semiconductors. Another MOF can instead break down harmful gases, including some that can be used as chemical weapons. Numerous companies are also testing materials that can capture carbon dioxide from factories and power stations, to reduce greenhouse gas emissions.
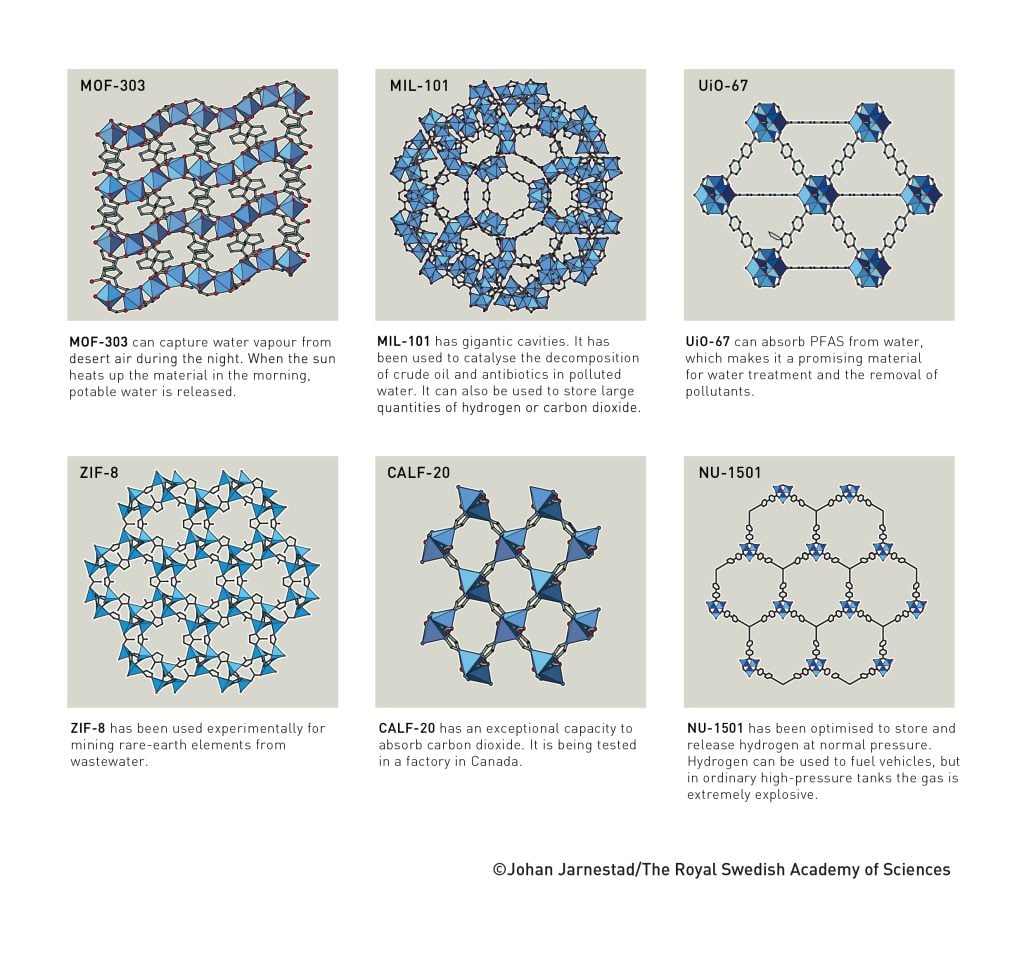
MOF-303 can capture water vapour from desert air during the night. When the sun heats up the material in the morning, potable water is released.
MIL-101 has gigantic cavities. It has been used to catalyse the decomposition of crude oil and antibiotics in polluted water. It can also be used to store large quantities of hydrogen or carbon dioxide.
UiO-67 can absorb PFAS from water, which makes it a promising material for water treatment and the removal of pollutants.
ZIF-8 has been used experimentally for mining rare-earth elements from wastewater.
CALF-20 has an exceptional capacity to absorb carbon dioxide. It is being tested in a factory in Canada.
NU-1501 has been optimised to store and release hydrogen at normal pressure. Hydrogen can be used to fuel vehicles, but in ordinary high-pressure tanks the gas is extremely explosive. ©Johan Jarnestad/The Royal Swedish Academy of Sciences
Some researchers believe that metal-organic frameworks have such huge potential that they will be the material of the twenty-first century. Time will tell, but through the development of metal-organic frameworks, Susumu Kitagawa, Richard Robson and Omar Yaghi have provided chemists with new opportunities for solving some of the challenges we face. They have thus – as Alfred Nobel’s will states – brought the greatest benefit to humankind.
Further reading
Additional information on this year’s prizes, including a scientific background in English, is available on the website of the Royal Swedish Academy of Sciences, www.kva.se, and at www.nobelprize.org, where you can watch video from the press conferences, the Nobel Prize lectures and more. Information on exhibitions and activities related to the Nobel Prizes and the prize in economic sciences is available at www.nobelprizemuseum.se.
The Royal Swedish Academy of Sciences has decided to award the Nobel Prize in Chemistry 2025 to
SUSUMU KITAGAWA
Born 1951 in Kyoto, Japan. PhD 1979 from Kyoto University, Japan. Professor at Kyoto University, Japan.
RICHARD ROBSON
Born 1937 in Glusburn, UK. PhD 1962 from University of Oxford, UK. Professor at University of Melbourne, Australia.
OMAR M. YAGHI
Born 1965 in Amman, Jordan. PhD 1990 from University of Illinois Urbana-Champaign, USA. Professor at University of California, Berkeley, USA.
“for the development of metal-organic frameworks”
Science Editors: Peter Brzezinski, Heiner Linke, Olof Ramström and Xiaodong Zou, the Nobel Committee for Chemistry
Text: Ann Fernholm
Translation: Clare Barnes
Illustrations: Johan Jarnestad
Editor: Alicia Hegner
© The Royal Swedish Academy of Sciences
Source link
