Autofluorescence suppression and high-sensitivity microscopy reveals nanoscale assemblies in human brain tissue
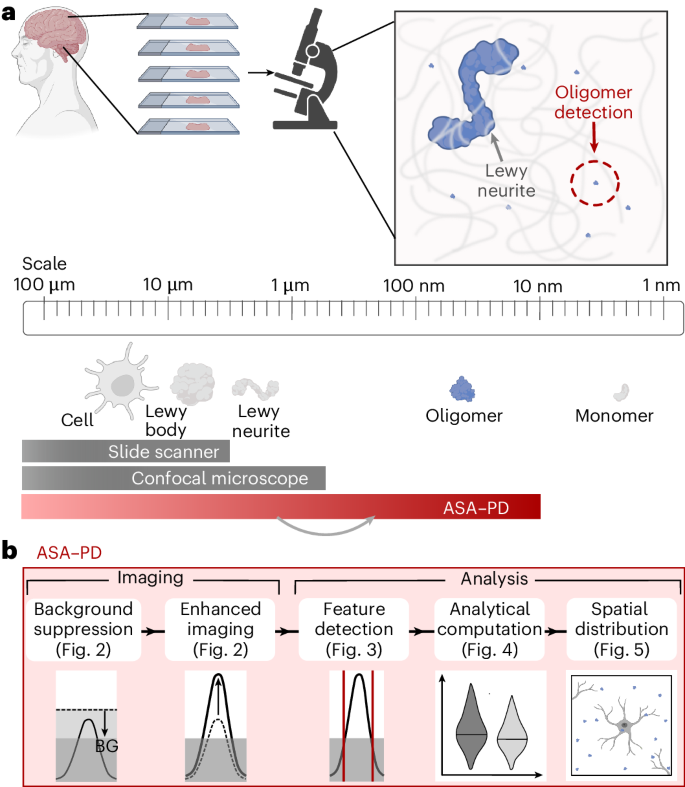
An overview of the ASA–PD pipeline is shown in Fig. 1. In brief, the aim is to capture spatial data over the entire scale of structure sizes most critical in PD, from individual cells to small aggregates (Fig. 1a). Detailed descriptions of the sample preparation steps are described in the Methods. First, 8-μm-thick brain tissue sections were mounted on glass slides, stained and then processed in the five stages illustrated in Fig. 1b: (1) background suppression, (2) enhanced imaging, (3) feature detection, (4) analytical computation and (5) spatial distribution analysis, where the first two steps contain the experimental portion of our workflow and steps 3–5 perform image-processing tasks and analysis.
a, ASA-PD is an imaging and analysis method for detecting protein aggregates in tissue down to nanoscale aggregates. b, The five main steps for imaging and analysis. Background suppression and enhanced imaging improve the signal-to-noise ratio such that aggregates can be detected and quantified in the analysis pipeline, including the spatial distributions relative to cell-specific markers. BG, background. Panels a and b created with BioRender.com.
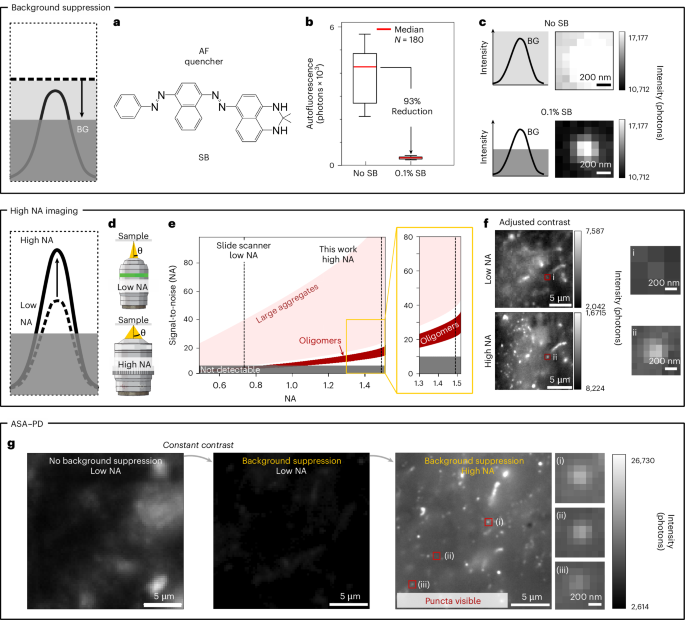
Observing protein aggregates is relatively routine in in vitro conditions38,39,40, but detecting small species in vivo poses a challenge owing to the poor signal-to-noise ratio in tissue. High background intensity, caused by tissue autofluorescence, acts as a noise floor that obscures the presence of dim objects such as oligomeric species. This noise effectively implements a brightness filter that leaves only large protein aggregates, with many attached fluorescent antibodies, as detectable species. To reduce the high autofluorescence of human brain tissue that inhibits sensitive imaging, we deployed Sudan Black B (Fig. 2a), a fat-soluble diazo dye and well-known autofluorescence quencher on untreated brain tissue sections41. Under optimized conditions, 10 min of incubation with 0.1% Sudan Black led to a 93% reduction in background autofluorescence for 561 nm laser excitation (26 W cm−2 illumination intensity), corresponding to a decrease in median detected photon counts from 4,400 ± 1,040 photons ± median absolute deviation (MAD) to 333 ± 47 photons (Fig. 2b) (the background reduction for other excitation colours (488, 561 nm), treatment times and concentrations are shown in Supplementary Fig. 1). Next, we repeated this background suppression step on antibody-labelled samples and evaluated various antibodies against multiple forms of α-synuclein for specificity and detectability (Supplementary Figs. 2 and 3 and Supplementary Table 1). The background reduction by Sudan Black facilitated the reliable detection of small features in images with a vastly improved signal-to-noise ratio for some of the antibodies tested, as shown in Fig. 2c.
a, Background suppression is achieved with the autofluorescence quencher Sudan Black (SB). b, Box plots showing the IQR and 5th–95th percentile bounds of autofluorescence (AF) intensity before (median of 4,400) and after treatment with 0.1% SB (median of 333). N = 180 images per sample. c, Before quenching, the fluorescence from Alexa Fluor 568 labelled small aggregates is masked by the background autofluorescence. After quenching, small aggregates can be easily visualized (both images excited at 561 nm, 26 W cm−2). d, A high NA objective collects a larger amount of light from the sample. e, The modelled signal-to-noise ratio for imaging punctate objects in post-quenched tissue background at 100× magnification across a range of NAs of objectives. Only at high NA (>1) large aggregates and oligomers become detectable. f, Images of p-syn stained PD tissue with 40× magnification, NA = 0.75 (top) and 100×, NA = 1.49 (bottom). Close-ups show that the same small aggregate is clearly visible at high magnification and high NA. g, Images of p-syn stained PD tissue with no background suppression and low NA (left), background suppression implemented and low NA (middle) and background suppression implemented and high NA (right). Several example puncta are shown in the closeups (oligomers) after background suppression is implemented and a high NA objective is used.
To visualize the α-synuclein aggregates most associated with PD, we used an antibody targeting phosphorylated α-synuclein at serine 129 (hereafter called p-syn). This post-translational modification promotes inclusion formation and/or toxicity in human cells42, Drosophila43 and rodent models44,45. Further, p-syn forms the vast majority of all insoluble α-synuclein aggregates in the PD brain46. Given this link between p-syn and pathology in synucleinopathies, we tested a variety of antibodies (Supplementary Table 1), including two complementary antibodies targeting the pS129 epitope of α-synuclein raised in two species (rabbit, AB_2270761, and mouse, AB_2819037). The AB_2819037 antibody showed characteristic Lewy pathology in both DAB and immunofluorescence staining and were shown to be specific through substantial co-localization with a second antibody for total α-synuclein (AB_2832854) (Supplementary Fig. 2). The final p-syn antibody selection, AB_2819037, was selected because of (1) the degree of coincidence of the p-syn antibody compared with total α-synuclein; (2) co-localization with other disease-related proteins, such as ubiquitin and p62; and (3) the demonstration of antibody specificity for human α-synuclein based on mouse tissue with the overexpression or knockout of human α-synuclein47. Furthermore, we confirmed (via electron microscopy and fluorescence imaging) that purified p-syn can aggregate in vitro, and form β-sheet rich 10-nm assemblies48. These recombinant p-syn protein aggregates can be detected by the same AB_2819037 antibody (Supplementary Fig. 4).
One way to improve the signal-to-noise ratio beyond reducing the overall background intensity is by improving the light-collection efficiency of the imaging system. In most microscopes, the least efficient step in light collection occurs at the objective lens of the microscope and is encoded in the numerical aperture (NA). Using a high NA objective lens has two main impacts: first, the NA scales with the collection angle of collected light49 (Fig. 2d and Supplementary equation (3)), and thus, more photons from the sample are collected at high NA. Second, increasing the NA improves the image resolution50 (Supplementary equation (8)). For imaging in tissue, we deployed a 1.49 NA oil-immersion, 100× microscope objective lens often used in single-molecule fluorescence applications51. The result is an overall increase in the signal-to-noise ratio for all objects, which is particularly important for the nanoscale assemblies that fall below the detectability range for lower NA objectives (Fig. 2e), such as the air objectives most often used in slide scanners for clinical applications52. Figure 2f compares a 0.75 NA 40× air objective lens (top) with the 1.49 NA 100× oil objective lens used in this study (bottom) for the same tissue sample stained for phosphorylated α-synuclein and quenched with 0.1% Sudan Black. The effect of background suppression and increased light collection using a high NA are shown in Fig. 2g. In these images, a wide variety of object sizes become visible; which we divide into two classes on the basis of their apparent size relative to the diffraction limit. Specifically, we define ‘large’ as greater than the optical diffraction limit of visible light, spanning ~200 nm to tens of microns, and ‘nanoscale’ as objects below the optical diffraction limit (<200 nm). The fluorescence signal from the latter manifests as small symmetric puncta in the image. Three examples of the nanoscale objects that become visible via ASA–PD are highlighted in Fig. 2g(i–iii). We refer to the latter objects as protein assemblies.
Applying the ASA–PD protocol within tissue revealed hundreds of detectable fluorescent puncta per field of view (FOV) (55 × 55 µm2) in both PD and HC samples (Supplementary Fig. 5). To perform statistically robust comparisons between samples, we developed a computationally efficient method for detecting and quantifying fluorescent species. This open-source analysis pipeline53 facilitates the rapid processing of large image libraries, facilitating transparent, shareable and verifiable results.
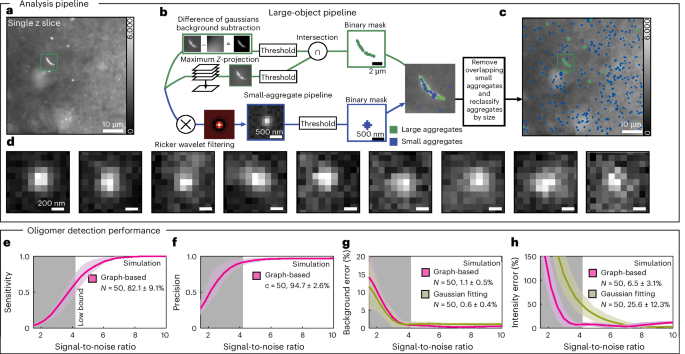
A schematic illustrating the analysis method and its validation is shown in Fig. 3. In brief, the analysis pipeline identifies features in an image, classifies them as either large aggregates or protein assemblies and quantifies details such as brightness, size and position53. A detailed description of the analysis is provided in Supplementary Information Note 3.1 and Supplementary Figs. 6–12. Figure 3a shows a typical PD image containing nano and microscale features. Microscale aggregates, such as Lewy bodies and Lewy neurites, are extremely bright in the dataset. These objects can be segmented with a simple intensity threshold after a background subtraction step (large-object pipeline in Fig. 3b and Supplementary Fig. 7). As large objects sometimes extend over multiple z-slices, the mask in each plane is multiplied by a segmented maximum-intensity projection from the z-stack. Smaller aggregates appear as dim, diffraction-limited puncta, and it is crucial to account for local background heterogeneity for detection (small-aggregate pipeline in Fig. 3b). To do so, we applied a bandpass filter to each image which selects features on the scale of the diffraction limit54 (Supplementary Figs. 6 and 9). Next, a threshold was used to create a mask containing only small objects. Objects with a footprint larger than the diffraction limit49 were reclassified as ‘large’ for subsequent analysis. Finally, the large and small aggregate masks were compared, and overlapping objects were removed from the nanoscale object dataset. Figure 3c shows an overlay of the detected objects on the original image, and a gallery of diffraction-limited puncta is shown in Fig. 3d.
a, A typical sample image containing features of various sizes and intensities, that is, Lewy neurites, micron-scale aggregates and sub-diffraction-limit oligomers. b, The aggregate detection pipeline for measuring large aggregates (top) and subdiffraction-sized features (bottom). The large object pipeline combines the z-projected intensity data with background-subtracted and threshold individual slices to generate a binary mask. Small aggregates are identified using a Ricker wavelet filter that acts as a bandpass, emphasizing small spots, which are then measured with a threshold and sorted by the number of pixels above the background. Features larger than the diffraction limit are reclassified as ‘large’ and features overlapping between the two masks are removed from the small aggregate pool. c, The large (green) and oligomer (blue) masks shown over the original image. d, The representative oligomers detected from c. e–h, The quantification of the pipeline performance using simulated images of diffraction-limited spots on a noisy background at various signal-to-noise ratios. The grey shaded region represents the lower quartile determined from experimental conditions, while the green and pink shaded area represents the mean ± s.d. The intensity and average background values for all detected peaks in simulated images were estimated by quantifying the pixel values around the detected peaks (pink curve) and by fitting a symmetric two-dimensional-Gaussian function with nonlinear least squares fitting (green). The presented values were obtained by averaging the mean and s.d. within the IQR of the experimental CNR data (Q1 = 4.2, Q3 = 8.1).
To evaluate the performance of the pipeline for detecting and characterizing nanoscale assemblies, we simulated images of puncta on noisy backgrounds at various signal-to-noise levels based on empirically determined parameters (Supplementary Note 3.2, Simulations). In the signal-to-noise range of our data, approximately ~4–8, the algorithm’s sensitivity was >82%, with a precision of >94% (Fig. 3e,f). At the same time, the relative error for estimating the local background per puncta outperformed nonlinear least-squares Gaussian fitting in this low signal-to-noise regime where Gaussian fitting performed poorly on aberrantly detected pixels, that is, false positives (Fig. 3g,h).
ASA–PD reveals a disease-specific shift in the nanoscale population of α-synuclein assemblies
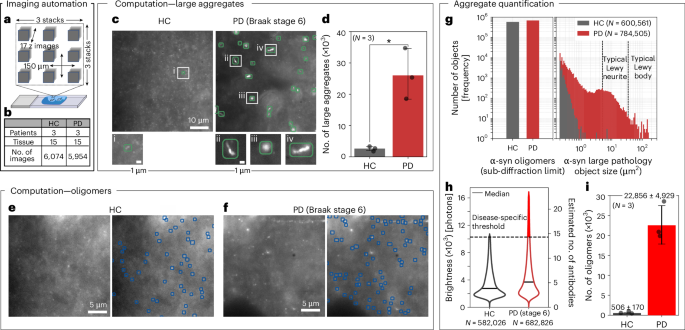
To characterize the distributions of α-synuclein in brain tissue, we selected three PD brains (Braak stage 6) and three HC brains for imaging (Supplementary Table 2). Tissue sections from the anterior cingulate cortical gyrus were put through the ASA–PD process. At this point, three principal regions within the grey matter were selected for investigation. At each of these regions, nine FOVs were captured in a 3 × 3 grid with a lateral separation of 150 μm to avoid any spatial overlap (each image covers 55 × 55 µm2). In total, 17 axial planes were recorded using a 500 nm step size (Fig. 4a and Methods). This process generated 13,770 high-resolution images (>41.6 mm2) that were manually validated to ensure the sample was in focus and the tissue contained no notable tears or defects. After this verification step, 12,028 images remained, 87.5% of the original dataset (5,954 PD and 6,074 HC images). These images were analysed as described in the previous section to map large aggregates (Fig. 4c,d) and nanoscale assemblies (Fig. 4e,f). Negative control samples, lacking primary antibodies, were also tested using PD tissue to quantify the degree of false positives caused by residual autofluorescence and unbound secondary antibodies (Supplementary Fig. 12).
a, The imaging of grey matter was performed in three areas, each area being a 3 × 3 grid of z stacks (17 slices) spaced 150 µm apart. b, The number of HCs and patients with PD (n = 3), number of tissue sections (n = 15) and number of images taken NHC = 6,074 and NPD = 5,954. c, Examples of analysed FOVs showing only the detected large aggregates. d, The number of large aggregates detected per patient over 1,800 FOVs (5.4 mm2). The mean ± s.d. for large aggregates was 3,866 ± 408 in HCs and 26,314 ± 7712 in PD, the means were compared by a two-tailed two-sample t-test, with P = 0.0147. e,f, Example FOVs of detected α-synuclein oligomers in HC and PD (Braak stage 6), respectively. g, The total number of α-synuclein aggregates in HC and PD tissues. The left panel shows oligomers (<0.04 μm2). The right panel shows large aggregates (>0.04 μm2). The typical Lewy neurites sizes (~5–30 μm2) and Lewy bodies (~30–300 μm2) are shown for reference. h, Violin plot of brightness of detected oligomers truncated at 1.5× IQR. Oligomers in HC had a median of 2,750 photons (MAD of 1,060) and of 3,700 photons (MAD of 1,690) for PD. The bright subpopulation of oligomers is shown in red for PD. i, The total number of detected oligomers per patient above this brightness threshold, 10,280 photons. Error bars are variation in boundary rejection percentage per patient, propagated. *P < 0.05.
From the ~12,000 images recorded across 30 tissue sections, we obtained a dataset containing more than 125,000 large aggregates and 1,260,000 nanoscale assemblies35,36. From the ~400 FOVs (~1.2 mm2), from each patient sample, the average number of large aggregates detected was ~tenfold higher in patients with PD than in the HC, with 26,314 ± 7712 in PD and 3,866 ± 408 in HC, respectively (Fig. 4d). These aggregates were distributed over a broad range of sizes from 0.04 to 100 µm2 in PD and 0.04 to 1 µm2 in HC (Fig. 4g), where the aggregate sizes associated with Lewy pathology essentially exclusively found in PD samples, consistent with the original tissue classifications (Supplementary Table 2). The total number of detected nanoscale objects in PD and HCs were much more similar (Fig. 4g), with 682,826 and 582,026 objects, respectively (with densities 0.082 objects per μm2 for PD and 0.067 objects per μm2 for HC).
While image resolution remains fundamentally diffraction-limited, the high sensitivity of ASA–PD to dim puncta, coupled with their relative sparsity, allows the detection of ~10 nm objects—far below the diffraction limit. At this scale, the resolution obscures aggregate sizes; however, for larger aggregates, where the size and brightness can be measured, the two were strongly linearly proportional, R2 > 0.99 (Supplementary Fig. 13a). We, therefore, characterized the distribution of nanoscale-object intensities as a proxy for the approximate size of these assemblies (Fig. 4h). The brightness distributions for all measured objects are shown in Supplementary Fig. 13b together with the estimated number of bound secondary antibodies, assuming each antibody contributes ~700 photons in our imaging conditions (Supplementary Fig. 14). Relative to the HC, the median was larger for PD samples, 3,700 photons (MAD of 1,690) and 2,750 photons (MAD of 1,060), and the distribution of brightnesses in PD samples was also broader, characterized by its interquartile range (IQR) IQRPD = 4,280 photons compared with IQRHC = 2,690 for HCs. To determine if the distribution tail was reproducibly different between PD and HC samples, we defined a brightness threshold using the HC measurements (Fig. 4h). The number of nanoscale objects above this threshold (10,280 photons, equating to ~15 bound secondary antibodies) is shown in Fig. 4i. This data represents ~10% of all measured PD assemblies but only 0.26% of those in HC (totalling 68,569 in PD and 1,518 in HC). The existence of this bright, disease-specific shift in the nanoscale population was highly robust by ASA–PD and was observed consistently when testing different α-synuclein antibodies, two different brain banks (Queen Square Brain Bank for Neurological Disorders (QSBB) and Multiple Sclerosis and Parkinson’s Brain Bank (Imperial), 12 individuals (6 PD and 6 HC), and using different antigen retrieval methods (formic acid and heat mediated epitope retrieval) (Supplementary Note 3.3, Supplementary Figs. 15–17 and Supplementary Tables 2 and 3).
Subpopulations of nanoscale α-synuclein assemblies are detectable by biochemical methods
Our approach enables the direct visualization of nanoscale aggregates that are typically challenging to detect. To further investigate the nanoscale assemblies revealed by our approach and determine if the fraction of bright nanoscale aggregates found in disease PD tissue could be detected with orthogonal methods, we performed PLA, enzyme-linked immunosorbent assays (ELISA), size exclusion chromatography (SEC) and seed amplification assays (SAAs) in brain tissue samples.
PLA can detect protein–protein interactions using antibody-linked DNA probes34. When these probes are adjacent, they produce an amplified signal that can be visualized using fluorescence microscopy (Supplementary Fig. 19a). This approach can be used to detect nanoscale assemblies of α-synuclein by amplifying the signal from α-synuclein 211 antibodies that are in close proximity. PLA was performed on 13 brain sections from the anterior cingulate gyrus, comprising six late Braak stage 5–6 PD and seven HC samples (Supplementary Table 3), and imaged (20× magnification, NA 0.75). Four images of the cingulate cortex were taken per sample and fluorescent puncta were quantified based on their intensity and a minimum size threshold of 0.9 µm. Representative images and the puncta density is shown in Supplementary Fig. 19b,c plotted per sample. The average number of puncta revealed an enriched population of aggregated α-synuclein in PD samples compared with HC samples, consistent with the ASA-PD data.
Next, brain lysates were fractionated using SEC to separate soluble α-synuclein species by apparent molecular weight from the Braak stage five or six PD, and HC donors (three PD and three HC; Supplementary Fig. 20a). This was followed by ELISA on the fractions to quantify the absolute amount of αSyn per fraction. Fractions were then subjected to the SAA, which allows the amplification and detection of small amounts of aggregates present in a sample. Recombinant human monomeric α-synuclein was used for the amplification of templated aggregation from pre-existing aggregates, and the kinetics of the amplification reaction was monitored by Thioflavin T (ThT) binding and increase in its fluorescence intensity.
The SEC-ELISA analysis revealed that total α-synuclein concentrations differed across high and low molecular weight fractions by two orders of magnitude but did not reveal statistical differences in the total α-synuclein in PD compared with controls, suggesting a similar amount of total protein (Supplementary Fig. 20b). The SAA revealed that high molecular weight fractions (200 kDa to 5 MDa) from PD brains had significantly shorter lag times, indicating the presence of seed competent αSyn aggregates, compared with samples derived from healthy brain tissue. Physiological (low molecular weight) fractions, in contrast, showed no statistical differences in lag times between PD and HC samples (Supplementary Fig. 20d,e). Comparisons between high molecular weight and low molecular weight fractions could not be made owing to altered α-synuclein concentrations. Therefore, there is a relatively small amount of aggregated α-synuclein (according to ELISA approx. 0.5% of total) both in physiological as well as disease tissue. In PD tissue, however, there is an apparent conversion of non-seed competent, or physiological, α-synuclein aggregates into seed competent aggregates.
Finally, we validated the presence of Proteinase K-resistant fluorescent puncta to assess whether the observed aggregates exhibited distinct chemicophysical properties (Supplementary Fig. 21). Notably, some bright puncta persisted after treatment, indicating a measurable degree of resistance to Proteinase K.
Collectively, the ASA–PD and amplification assay data confirm the presence of a population of small, soluble protein assemblies in the brain, which changes in PD samples, namely a subpopulation of bright assemblies has distinctive chemicophysical properties, such as their size and seed competence, and Proteinase K resistance.
Disease-specific assemblies are spatially heterogeneous
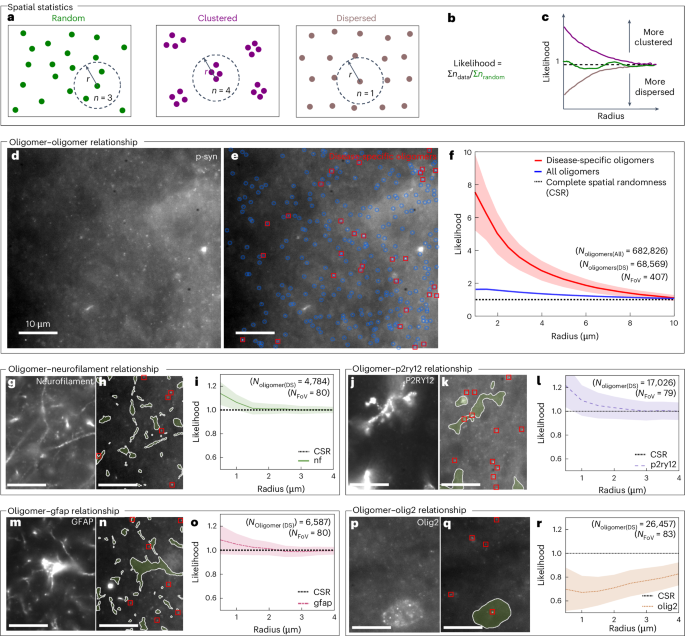
In addition to measuring object densities and size distributions, ASA–PD can be used to analyse spatial distributions (Fig. 5). To determine if the heterogeneity reported for larger α-synuclein aggregates4,55 extends to nanoscale species in PD samples, we performed a spatial-clustering test, which compares the likelihood of encountering assemblies as a function of the distance from it53 (Fig. 5a–c). Over 400 FoVs, 682,826 nanoscale objects were detected and characterized. On average, these species were found to cluster relative to a complete spatial random distribution. (Fig. 5f, blue); however, the disease-specific populations exhibited a substantially higher degree of clustering (68,569 aggregates; Fig. 5f, red).
a, An illustration showing example spot patterns with random, clustered and dispersed underlying spatial distributions. b, The equation used here for likelihood calculation. c, An example likelihood plot showing likelihood as a function of radius for random, clustered and dispersed spatial distributions. d, An example of an analysed FOV with an antibody stain for p-syn. e, The same analysed FOV with detected oligomers in blue and red, with red serving to highlight the disease-specific oligomers. f, A plot showing the likelihood of oligomer–oligomer distances, with 95% confidence interval presented as the shaded region, shows that all oligomers tend to spatially cluster, and that disease-specific oligomers have a higher clustering tendency. g–r, Example FOVs stained with antibodies for neurofilaments, P2RY12, GFAP and Olig2 (g, j, m and p), the same images with labelled cells shown in green and disease-specific oligomers highlighted in red (h, k, n and p) and a plot showing the likelihood of oligomer–cell distances, with the 95% confidence interval presented as the shaded region, which shows that disease-specific oligomers tend to cluster in or around neurons, microglia and astrocytes, while being dispersed from oligodendrocyte nuclei (i, l, o and r).
ASA–PD can also be used to interrogate the distance of objects to cell-specific markers in co-stained samples. To do so, we co-stained samples with α-synuclein and various cell markers, minimizing photobleaching and optimizing the signal of the nanoscale species. We then quantified the density as a function of the proximity to the cell markers (Fig. 5g–r). For some cell types, namely neurofilament (neurons), P2RY12 (microglia) and GFAP (astrocytes), the disease-specific species showed an increased probability of being localized in proximity to neurofilament (neurons), P2RY12 (microglia) and GFAP (astrocytes). By contrast, disease specific species have a low probability of being localized to the Olig2 marker (oligodendrocyte nuclei). This approach can thus enable quantitative statistical analysis of differences of disease-specific species density over large data sets.
Source link