For some three billion years, unicellular organisms ruled Earth. Then, around one billion years ago, a new chapter of life began. Early attempts at team living began to stick, paving the way for the evolution of complex organisms, including animals, plants and fungi.
Across all known life, the move to multicellularity happened at least 40 times, suggests one study1. But, in animals, it seems to have occurred only once.
Beginning in the early 2000s, researchers interested in this remarkable event made a series of unexpected discoveries. The prevailing view held that a flood of genes had to evolve to enable the key properties of multicellularity2: the ability of cells to stick together, communication using molecular signals and the coordinated regulation of gene expression that causes each cell to specialize and take its position in the organism. But studies found that some unicellular organisms express a slew of proteins that control key properties of multicellularity in animals3,4. The molecular toolkit required for multicellularity seems to have existed well before the first animals came to be.
“This work has rewritten our understanding of animal origins,” says William Ratcliff, an evolutionary biologist at the Georgia Institute of Technology in Atlanta. “And it makes us ask different questions.”
The two teams behind much of this research were led by evolutionary biologist and geneticist Nicole King at the University of California, Berkeley, and by evolutionary biologist Iñaki Ruiz-Trillo at the Institute of Evolutionary Biology in Barcelona, Spain. They have since expanded into a small community of scientists that has developed more than a dozen of these species into model organisms. All of these species are eukaryotes, which are distinct from prokaryotes in that they have a nucleus, and belong to lineages closely related to animals: choanoflagellates, filastereans, ichthyosporeans and corallochytreans (see ‘Animals’ unicellular relatives’). Many of the model species dabble in multicellularity by occasionally forming colonies.
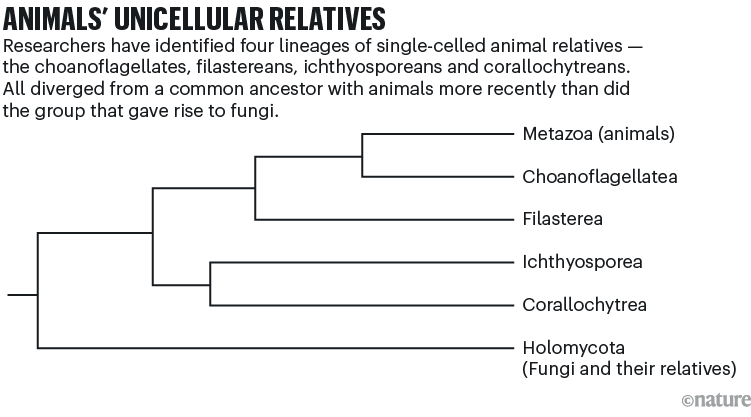
Source: Ruiz-Trillo, I. et al. Annu. Rev. Microbiol. 77, 499–516 (2023).
Part of what makes these organisms so interesting is how different they are — in appearance, life stages and genetic make-up — researchers say. Each of the organisms, five of which are featured here, offers a peek into the evolutionary paths that could have led to animals. Looking across several lineages to piece together this event has become “the philosophy of this scientific community”, says molecular biologist Elena Casacuberta, who jointly runs the laboratory in Barcelona with Ruiz-Trillo. “Only with a comparative approach can we attempt to have a more accurate picture.”
The top model
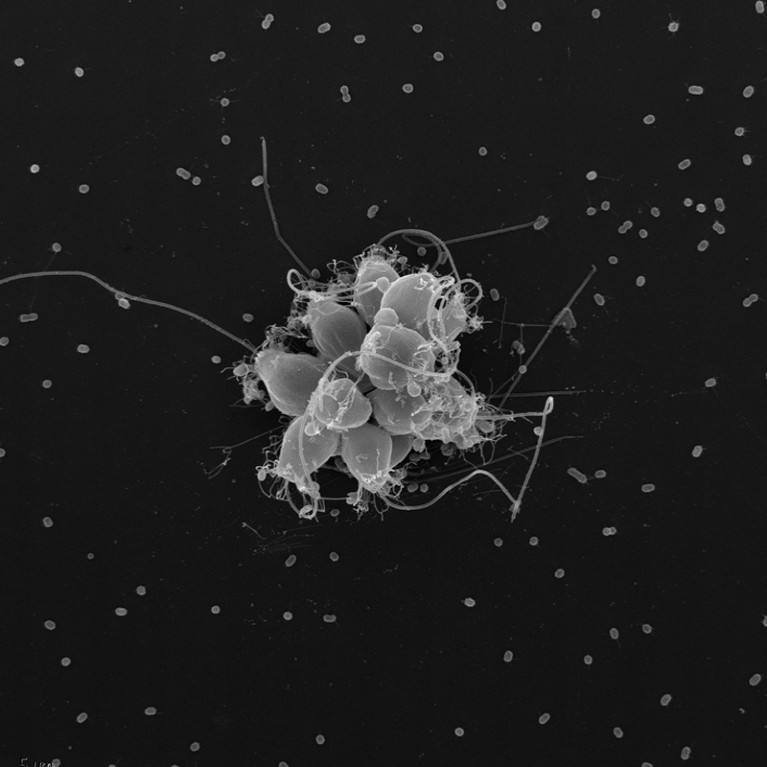
When the single-celled choanoflagellate Salpingoeca rosetta divides in the presence of bacteria, its daughter cells form a rosette pattern.Credit: Mark Dayel
Salpingoeca rosetta was among the organisms that King investigated in her early work on multicellularity. It belongs to the choanoflagellates, which are the closest living relatives of animals. This group diverged from a common ancestor with animals more than 600 million years ago.
Like other choanoflagellates, S. rosetta has a spherical cell body that sports a collar of thin membrane protrusions called microvilli, which are used to capture bacteria swept up for dinner by a long tail (known as a flagellum). It was isolated in 2000 from mudflats off the coast of Virginia; researchers haven’t managed to find it again in the wild. Under certain environmental conditions, S. rosetta cells divide clonally, producing genetically identical daughter cells that form colonies by circling up in a rosette pattern, with flagella undulating outward. But when King first started working with the organism in the lab, she could not budge it from its unicellular form. A chance experiment revealed that secretions by a specific prey bacterium act as a signal for the cells to start dividing.
A ‘lost world’ of early microbes thrived one billion years ago
In addition to forming rosettes, the organism has at least one other multicellular conformation and a few distinct free-living cell types. When confined to a tight space, for example, S. rosetta cells withdraw their flagella and become amoeboid, lacking a firm shape and extending slender tentacles called filopodia to pull themselves along.
“It has such an amazing diversity in response to a myriad of environmental cues,” says David Booth, a biochemist at the University of California, San Francisco.
In 2003, King and her colleagues reported the presence of proteins involved in cell adhesion and cell signalling in choanoflagellate species3. They later identified a more in-depth toolkit for multicellularity when they sequenced the genome of S. rosetta5.
Salpingoeca rosetta is the Drosophila of choanoflagellates — the most widely studied species and the one for which researchers have developed the most extensive tools for directly altering the genome. In 2018, during a postdoc in King’s lab, Booth and his colleagues managed to add DNA encoding fluorescent proteins into S. rosetta6 and, in 2020, found ways to edit its genome using CRISPR7. Such methods have made it possible to tinker directly with genes that researchers say could be key to multicellularity — and to study the proteins that those genes express.
With the tools’ help, microbiologist Arielle Woznica at the University of Texas at Austin, a former graduate student in King’s lab, is exploring how choanoflagellates respond to bacterial attackers. Biochemist Florentine Rutaganira, a former postdoc in King’s lab now at Stanford University in California, is investigating enzymes called tyrosine kinases — linchpins of cell signalling in animals that are present in similar numbers in S. rosetta.
Master aggregator

Environmental cues can trigger aggregation in Capsaspora owczarzaki.Credit: H. Suga et al./Nature Commun.
Capsaspora owczarzaki is a member of the filasterean lineage, which diverged from a common ancestor with animals perhaps a couple of hundred million years earlier than did choanoflagellates.
Ruiz-Trillo first became interested in the organism as a postdoc, just as King’s lab was setting up to study S. rosetta. He and his colleagues found that C. owczarzaki, too, seemed to be closely related to animals4. Ruiz-Trillo set out to study lineages other than choanoflagellates, because investigating several lineages could provide a fuller picture of the shared ancestor with animals. After sequencing the genome of C. owczarzaki, Ruiz-Trillo and his team reported in 2013 that it has many genes related to multicellularity, including some that choanoflagellates lack — such as those that encode cell-surface proteins called integrins, which help cells to stick to each other and to their environment8.
These bizarre ancient species are rewriting animal evolution
Discovered in 2002 inside a freshwater snail, C. owczarzaki spends most of its life cycle as a unicellular amoeba, but environmental cues can push the cells into a multicellular phase in which clusters swarm together and fuse into increasingly large aggregates. This path to multicellularity is different from the clustering through clonal division observed in choanoflagellates. Ruiz-Trillo says that clonal division is “the standard way in which people were thinking animals evolve”.
During development in animals, a single cell divides into many cells with identical genomes, a process that probably avoids any genetic conflict arising between cells. It’s therefore an easy leap to assume that clonal division was also the evolutionary path to the first animals, Ruiz-Trillo says. But aggregation exists in many eukaryotic lineages as a fast and easy way for cells to form 3D structures, and he thinks this mechanism deserves a closer look.
Ruiz-Trillo and his colleagues found that C. owczarzaki uses some key genes related to multicellularity during this aggregated phase9. Perhaps aggregation was the essential step in the evolution of animals, Ruiz-Trillo says, or perhaps it was just one part of the process.
Capsaspora owczarzaki is one of a handful of unicellular species that Casacuberta and Ruiz-Trillo happily send to other labs on request.
The dualist
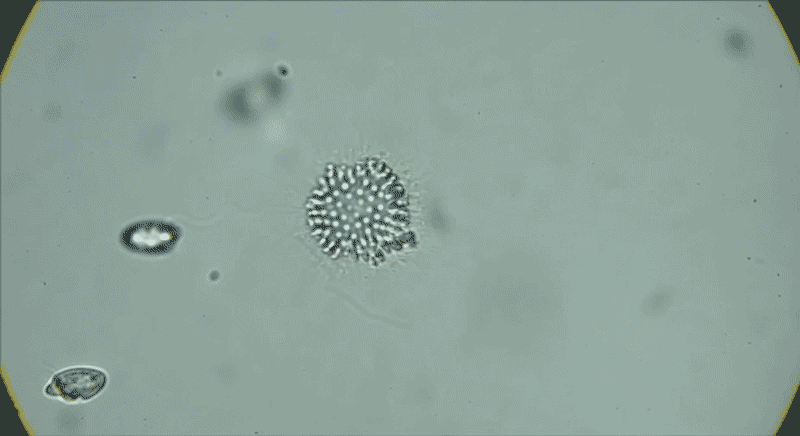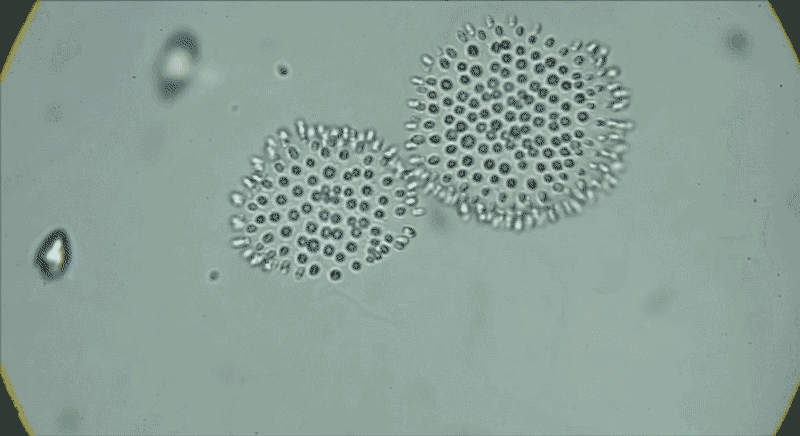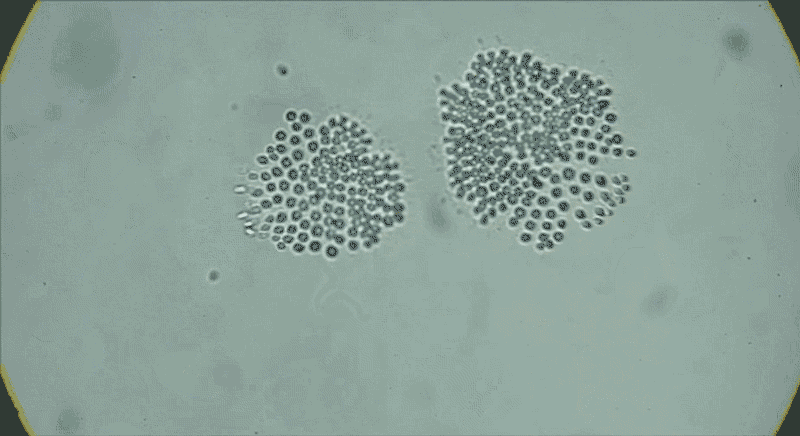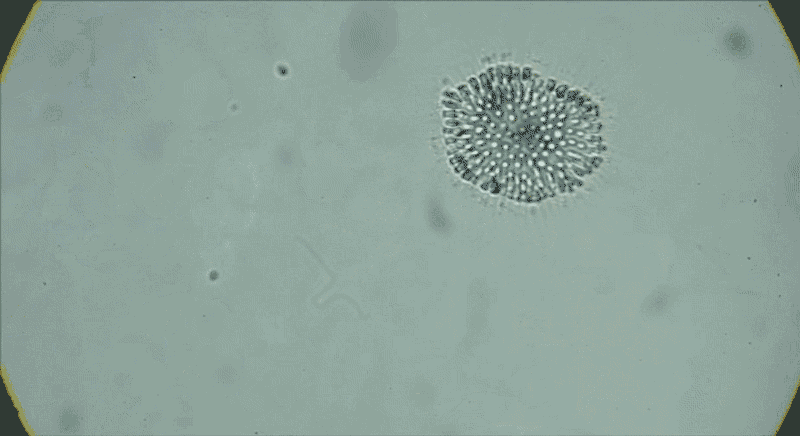
In water from a tide pool, a colony of Choanoeca flexa cells that has taken on a cupped shape reverses its curvature. Another colony joins it, and their curvatures reverse again.Credit: Benjamin T. Larson
The serendipitous discovery in 2017 of another species of choanoflagellate, Choanoeca flexa, demonstrated how much variation exists in this group alone. Thibaut Brunet, an evolutionary cell biologist now at the Pasteur Institute in Paris, found C. flexa in Curaçao, with his colleagues while attending a workshop as postdoc in King’s lab. After collecting water samples from shallow marine tide pools while touring the island, he and his colleagues gaped at the scene under the microscope.
Individual cells, which look much like S. rosetta cells, form a cupped monolayer sheet, with all the flagella pointing in the same direction10. In response to light or darkness, “they could reverse their curvature in a few seconds, flipping inside out like a child’s toy or an umbrella”, says Brunet. “We were screaming and jumping up and down when we saw it — we were probably ridiculous.”
The secret lives of cells — as never seen before
Although researchers are still developing methods to manipulate its genome, C. flexa has at least one big benefit as a model organism. Other organisms have been studied only in the lab after they were discovered, says evolutionary biologist Núria Ros-Rocher, a postdoc in Brunet’s lab. Researchers don’t know how or where to find them again. But C. flexa has been retrieved repeatedly from the tide pools in which it was first found. “We were very lucky, because we could go back to the natural environment to understand how it’s linked to the organism’s multicellular behaviour,” says Ros-Rocher.
Those pools face whiplash changes — water often heats up and evaporates within days, leaving the organism afloat in sharply elevated salinity or beached on dried mud before the tide submerges it again. Brunet and his team found that C. flexa, like S. rosetta, can go from a unicellular to multicellular state by clonal division. But it can use aggregation, too, and sometimes combines both strategies at once.
Source link