A pioneering new liver cancer treatment, employing focused ultrasound waves to destroy diseased tissue, has been administered to the first NHS patient.
Addenbrooke’s Hospital in Cambridge is the first in Europe to provide this procedure, known as histotripsy, outside of clinical trials.
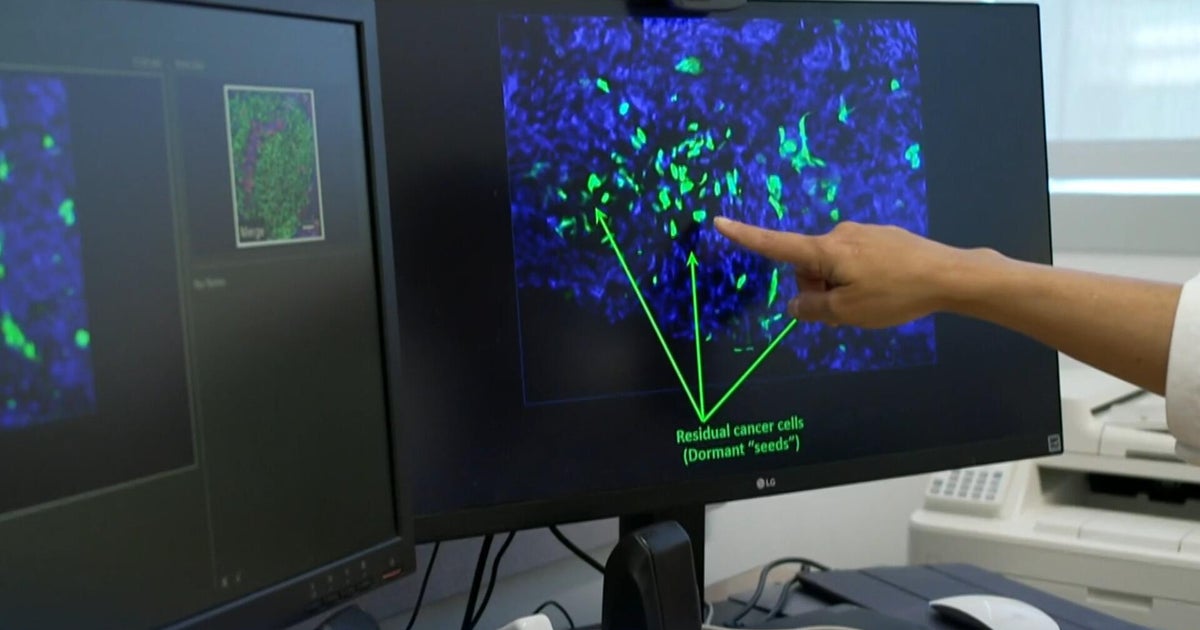
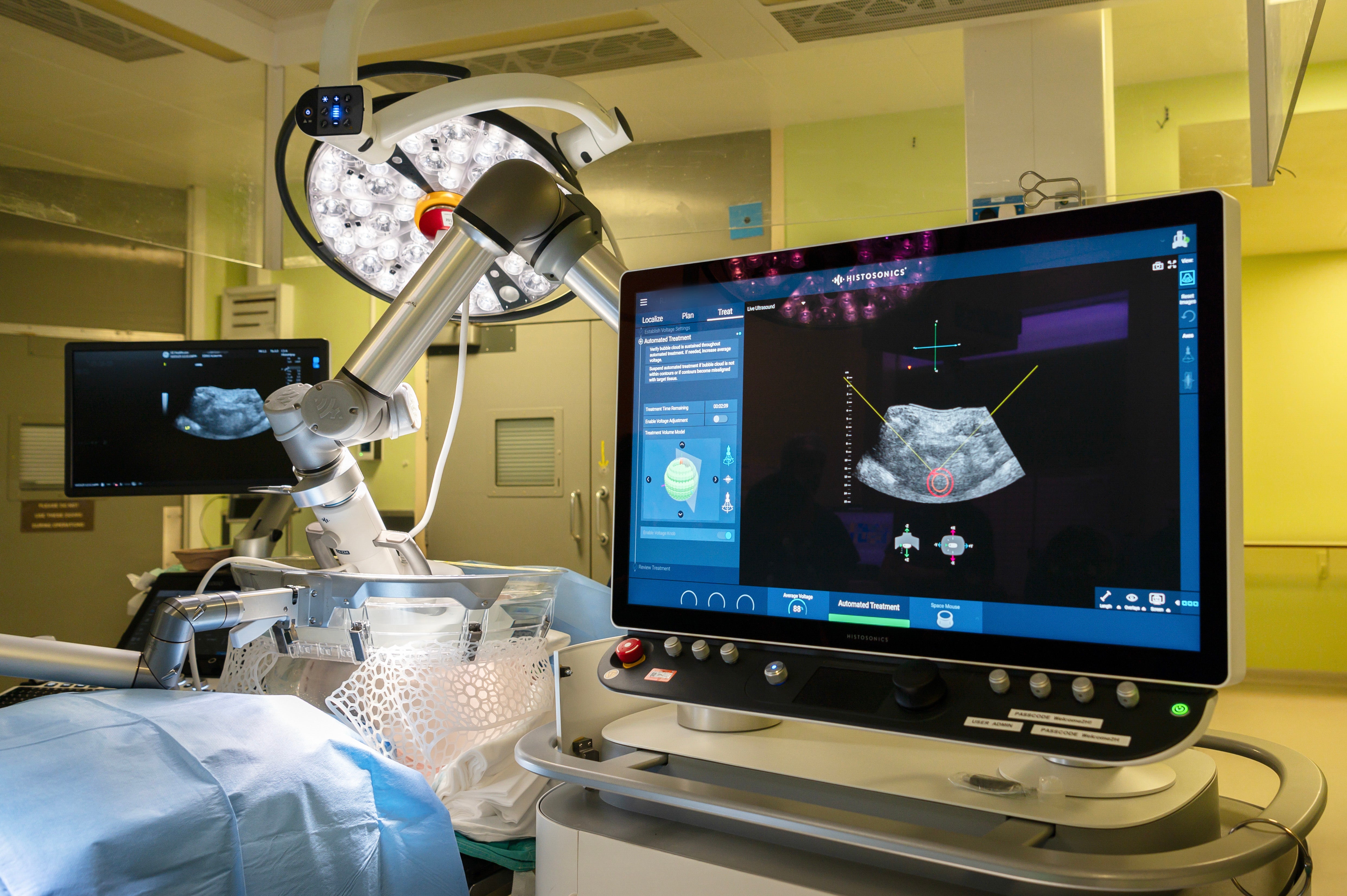
The innovative method works by using ultrasound to create microscopic bubbles from gases within tumour tissue, which rapidly form and collapse in microseconds to destroy cancerous cells.
This non-invasive approach takes as little as 30 minutes, crucially avoiding surgery, radiation, or chemotherapy, meaning patients spend less time in hospital.
The histotripsy was performed on Roger Jackson, 80, at Addenbrooke’s Hospital in Cambridge.
Dr Teik Choon See, consultant interventional radiologist at Cambridge University Hospitals NHS Foundation Trust (CUH), led the procedure, which took place earlier this month.

He said: “Histotripsy represents a major and exciting step forward in cancer treatment.
“It allows us to target tumours more precisely while sparing surrounding healthy tissue, offering patients a safer and faster alternative to traditional therapies.
“What is even more promising is in some reported cases, after the sound waves break apart the tumour, the patient’s immune response may become activated and clear up some remaining cancerous tissues, showing real hope for patients.”
Mr Jackson, a retired sales manager from Bedford, was discharged the day after the histotripsy.
He said: “I feel privileged to be the first NHS patient and to receive this care was an amazing experience.
“It is impressive to think that sound waves can treat cancer, without the need for patients like me to go through intensive surgery, at what already is a stressful time.
“I’m hugely grateful to the team at Addenbrooke’s for their specialist care and expertise.”
Mr Jackson’s treatment is the first to be performed after the histotripsy technology was fast-tracked and granted unmet clinical need authorisation (Ucna) in Great Britain under the Innovative Devices Access Pathway.
Ucna, which is overseen by the Medicines and Healthcare products Regulatory Agency (MHRA), gives access to medical devices under certain conditions before full regulatory approval.
Addenbrooke’s will initially offer treatment to selected patients with tumours from primary and secondary liver cancers.
Roland Sinker, chief executive of CUH, said: “Histotripsy represents a hugely exciting and new era of cancer innovation and care.
“With faster recovery times and shorter hospital stays, this not only reduces the strain on our hospital beds, but it also frees up surgeons to focus on the more complex cancer cases, helping to cut waiting times.”
Health Secretary Wes Streeting said the move “marks the beginning of a new generation in cancer treatment”.
“We are lighting the fuse beneath the technological revolution, transforming care for NHS patients,” he added.
“By slashing red tape, we’ve made sure this game-changing new cancer treatment has reached the NHS front line quicker, and I’m proud to say British patients are now the first in Europe to benefit.
“This government has streamlined approval processes to create an NHS fit for the future, protecting patients while unleashing the full potential of our scientists and NHS staff so they can deliver world-class care.”
The histotripsy technology was developed in the US and has treated more than 2,000 patients worldwide since being granted Food and Drug Administration approval for the destruction of liver tumours in 2023.
Studies are under way to explore its effectiveness on other cancer types.
Source link