Gray hair is nothing to be ashamed of. In fact, according to a new study by researchers in Japan, the presence of gray hair may be a good sign that your body is naturally protecting itself from cancer.
A series of mouse experiments suggests we’ve evolved to let go of cells that are at risk of generating tumors at the expense of a little color.
Our cells are routinely subjected to a barrage of ‘genotoxic insults,’ or DNA damage wrought by a wide variety of environmental factors. Skin cells bear the brunt of many such affronts, given their role in helping buffer our internal organs from the outside world.
Related: Does Stress Really Turn Your Hair Gray?
DNA damage can contribute to cell aging as well as to the development of cancer, although the specific genotoxins, signals, and cellular mechanisms involved with the physical signs of aging remain poorly understood.
The new study focuses specifically on melanoma, a type of cancer predominantly found in the skin, where it originates in melanocytes – specialized skin cells that generate melanin, the pigment responsible for skin and hair color.
Melanocytes themselves arise from stem cells (McSCs) located within the hair follicles of mammalian skin, where they maintain skin and hair pigmentation via regular regeneration.
Using mice, the researchers profiled the expressions of the tissue’s genes to uncover the fates of the McSCs subjected to various kinds of DNA damage.
In the case of damage known as a double-strand break, in which both strands in the DNA’s double helix are severed, the researchers identified a specific response.
The McSCs irreversibly differentiated and disappeared, resulting in the mouse’s hair turning gray.
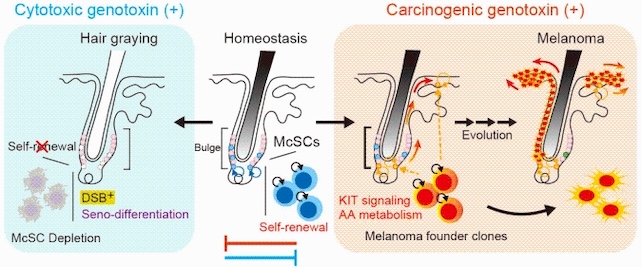
Known as senescence-coupled differentiation, or ‘seno-differentiation,’ this process relies on activation of the p53-p21 signaling pathway, which helps regulate cell cycling.
On the other hand, certain carcinogens triggered a remarkably different response. Researchers treated the skin of mice with ultraviolet B (UVB) light and 7,12-dimethylbenz(a)anthracene (DMBA), a potent carcinogen often used to induce tumor growth in laboratory research.
When exposed to these carcinogens, McSCs bypassed the differentiation process that occurred after double-strand breaks, even if the cells had suffered DNA damage, the study found.
Despite the general benefits of resilience, there is also something to be said for knowing when to quit. For McSCs, bowing out in response to DNA damage may be worth the loss of hair color if it means a lower risk of skin cancer.
When exposed to UVB or DMBA, melanocyte stem cells retained their self-renewal abilities and continued to clone themselves, the researchers report. This effect is supported by a cytokine called stem cell factor (SCF), which is involved with guiding the melanocytes to their proper place in the skin.
Secreted in the stem cell‘s local microenvironment, SCF also suppresses seno-differentiation. Instead of containing DNA damage, this increases the risk of tumors developing by encouraging compromised McSCs to carry on.
“These findings reveal that the same stem cell population can follow antagonistic fates – exhaustion or expansion – depending on the type of stress and microenvironmental signals,” says lead author Emi Nishimura, a biologist at the University of Tokyo.
“It reframes hair graying and melanoma not as unrelated events, but as divergent outcomes of stem cell stress responses,” she adds.
This does not mean gray hair is itself a defense against cancer risk, the researchers note. Graying is a result of seno-differentiation, a protective pathway that helps the body respond to genotoxic stress by eliminating potentially dangerous cells.
When this process doesn’t take place, however, the resulting survival and proliferation of damaged McSCs may favor melanoma.
More research will be needed to shed light on the mechanisms involved and to investigate corresponding phenomena in humans, but this already represents a significant leap in understanding.
With this insight into the molecular circuits governing McSCs’ divergent fates, the study presents a model to help explain key details about the relationship between tissue aging and cancer, the authors write.
The study was published in Nature Cell Biology.
Source link


