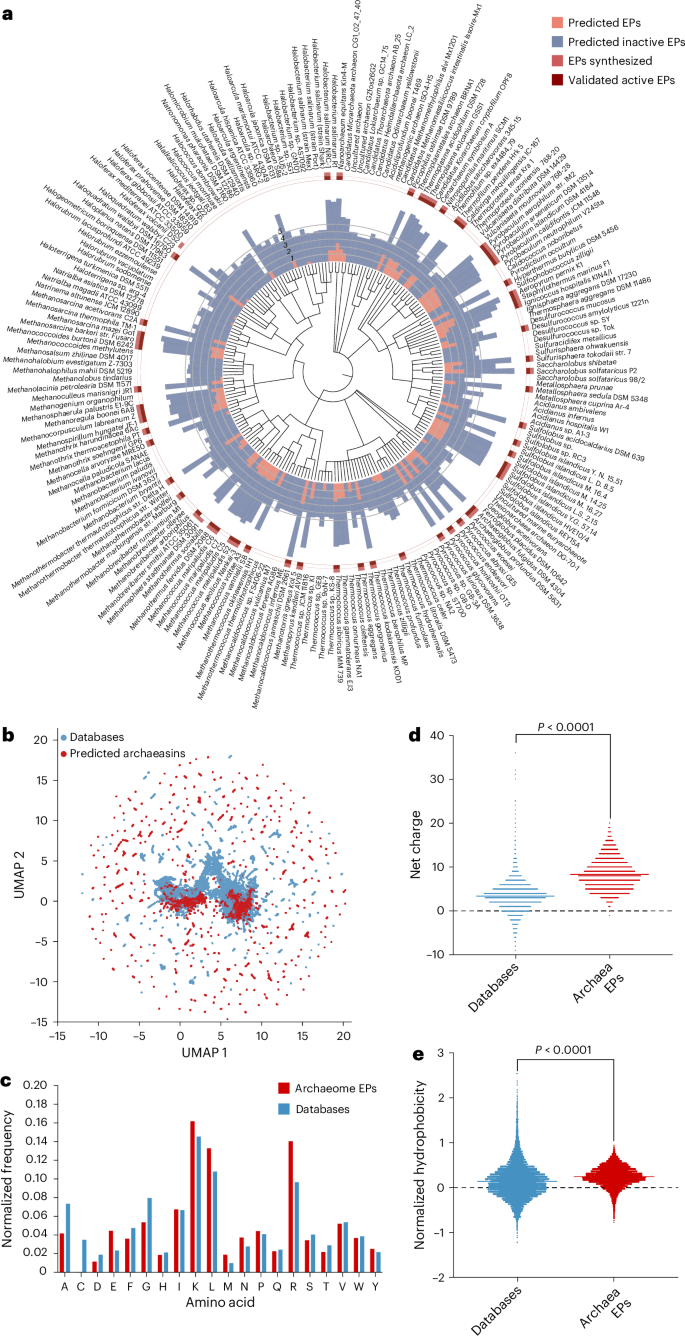
Deep-learning-guided identification of archaeasins
We collected 18,677 non-redundant reviewed protein sequences from 233 archaeal organisms available on UniProt10 and used APEX 1.1, a deep learning antimicrobial activity predictor6 retrained on updated data (‘APEX 1.1’ in Methods), to mine EPs within archaeal proteomes. As APEX predicted bacterial-strain-specific minimum inhibitory concentrations (MICs), we used the mean MIC to represent the overall antimicrobial potency of the peptides and found 12,623 EPs with a mean MIC ≤100 μmol l−1 (Fig. 1a and Supplementary Data 1), representing an archaeasin ratio of 0.00653% from the 193,331,608 archaeal peptides scanned. To assess whether this signal exceeded random expectations, we generated a non-redundant set of 193,288,387 randomly sampled peptides with a length distribution matching that of the archaeasins. Applying the same AMP criterion (mean MIC ≤ 100 μmol l−1), we identified 5,292 predicted active peptides from the random set, corresponding to an AMP rate of 0.00274%, or roughly 2.38× lower than that observed in archaeal EPs. These findings suggest that antimicrobial sequences are statistically enriched in archaeal proteomes relative to random sampling.
a, Archaeal proteomes were systematically scanned to identify EPs with potential antimicrobial activity. Circular bars denote the log10-transformed average active (red) and inactive (blue) EPs discovered by APEX. A peptide was classified as active if its predicted mean MIC against tested bacterial strains was ≤100 μmol l−1. The values were normalized by the number of proteins per organism scanned. Archaea with peptides that were synthesized are indicated by a light red square, and those experimentally validated as active are highlighted with a dark red square. b, Sequence space exploration using a similarity matrix. The graph illustrates a bidimensional sequence space visualization of peptide sequences found in DBAASP and antimicrobial EPs discovered by APEX in archaea organisms. Sequence alignment was used to generate a similarity matrix for all peptide sequences in DBAASP and the 12,623 antimicrobial EPs predicted by APEX (Supplementary Data 1 and 2). Each row in the matrix represents a feature representation of a peptide based on its amino acid composition. UMAP was applied to reduce the feature representation to two dimensions for visualization (Extended Data Fig. 2a). c, Comparison of amino acid frequency in archaeal EPs with known AMPs from the DBAASP, APD3 and DRAMP 3.0 databases (Extended Data Fig. 2b–e). d,e, Distribution of two physico-chemical properties for peptides with predicted antimicrobial activity, compared with AMPs from DBAASP, APD3 and DRAMP 3.0: net charge (d) and normalized hydrophobicity (e). Net charge influences the initial electrostatic interactions between the peptide and negatively charged bacterial membranes, whereas hydrophobicity affects interactions with lipids in the membrane bilayers (Extended Data Fig. 2). The Chi-squared test of independence of variables in a contingency table was used to compare the amino acid composition in c; P values were 0, that is, below machine precision levels, suggesting that they are statistically significant. Statistical significance in d and e was determined using two-tailed t-tests followed by a Mann–Whitney test; P < 0.0001. The solid line inside each box represents the mean value for each group.
We next examined whether archaeal species with larger genomes tend to encode a greater number of predicted AMPs. To do this, we compiled the number of predicted active EPs (defined as peptides with a mean MIC ≤100 μmol l−1 across 11 pathogen strains) and the total number of protein-coding genes, based on National Center for Biotechnology Information (NCBI) annotations, for the 10 archaeal genera with the highest and lowest EP counts. This dataset enabled us to assess potential relationships between genomic content and AMP abundance (Supplementary Table 1). A Spearman correlation analysis revealed a statistically significant positive correlation between genome size and the number of predicted EPs (Spearman’s rank correlation coefficient (ρ) = 0.4475, P = 0.0479), suggesting that archaeal species with larger genomes may harbour a broader repertoire of latent antimicrobial sequences.
Interestingly, several of the top-scoring genera, such as Pyrococcus, Methanocaldococcus, Pyrobaculum and Sulfolobus, are known thermophiles. This observation raises the possibility that certain lifestyle traits, such as adaptation to high-temperature environments, may be associated with greater bioactive peptide abundance. Although this analysis focused primarily on genome size, we acknowledge that environmental factors such as temperature tolerance, metabolic specialization or ecological niche may also influence the diversity and prevalence of encrypted AMPs. However, we consider this a preliminary analysis and expect that, as more archaeal genomes are catalogued and annotated, the observed trends will become more robust and enable more detailed exploration of ecological and evolutionary correlates of active peptide abundance.
To investigate the distribution of AMP-like EPs within the archaeal proteomes, we first performed sequence alignment on the combined set of 12,623 EPs and 19,775 publicly available AMPs from DBAASP11, APD3 (ref. 12) and DRAMP13 (see ‘APEX 1.1’ in Methods). We then applied uniform manifold approximation and projection (UMAP)14 to reduce and visualize the sequence similarity matrix derived from the local sequence alignment (Fig. 1b and Extended Data Fig. 2a).
Focusing on the top 265 archaeasins (mean MIC < 80 μmol l−1; Supplementary Data 2) that are sequentially diverse (see ‘Archaeasin selection’ in Methods), we analysed their source proteins by retrieving Gene Ontology annotations (Extended Data Fig. 2b). Gene Ontology term frequencies revealed that many of these top-ranking archaeasins originated from cytoplasmic proteins and proteins with essential cellular functions, including ATP binding, metal-ion binding, DNA binding, tRNA binding and zinc-ion binding. Several archaeasins were also derived from structural ribosomal proteins, plasma membrane proteins and proteins involved in translation. Collectively, these findings highlight the broad distribution and functional diversity of archaeasins throughout archaeal cells.
The amino acid composition of archaeasins revealed distinctive features compared with known AMPs from databases (Fig. 1c) and EPs previously discovered in the human proteome using APEX6 and a complementary scoring function4,7 (Extended Data Fig. 2c–f). Archaeasins were notably enriched in glutamic acid residues, surpassing levels typically found in known AMPs. This higher prevalence of negatively charged residues is also observed when comparing archaeasins with other EPs from human proteins. Nonetheless, archaeasins maintain a prevalence of cationic residues, leading them to display a slightly higher proportion of cationic residues compared with database entries, suggesting a unique balance in charge distribution (Fig. 1d). Despite these differences, their hydrophobicity remains comparable to standard database sequences (Fig. 1e). In addition, archaeasins show a tendency towards increased amphiphilicity, indicating a balanced distribution between hydrophobic and hydrophilic residues (Extended Data Fig. 3 and Supplementary Table 2). This analysis supports the notion that archaea, like humans7, encode a rich and compositionally unique repertoire of antimicrobial EPs, highlighting their potential as an unusual source of antibiotics and providing an evolutionary contrast that aids in deciphering antimicrobial diversity across domains of life.
Antimicrobial activity of archaeasins against bacterial pathogens
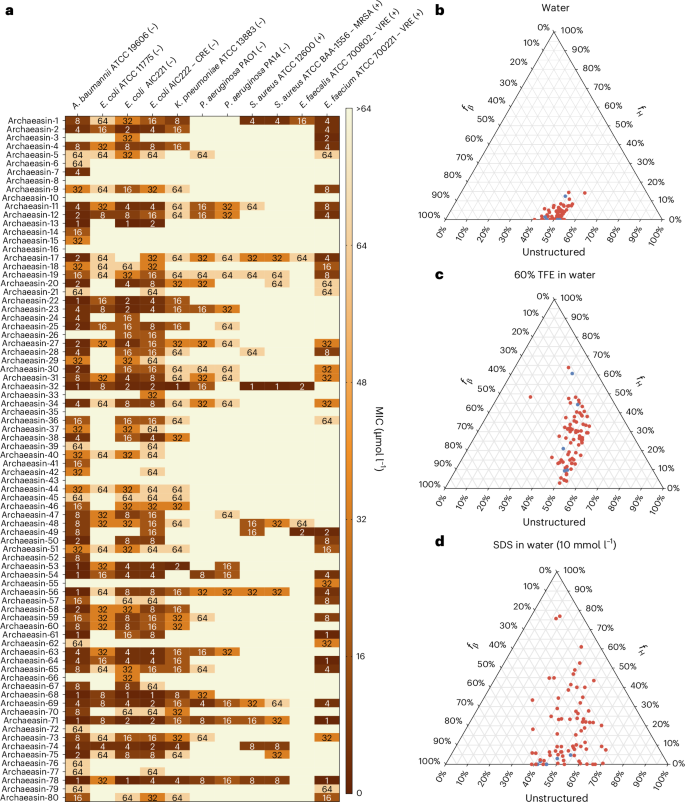
To experimentally validate the antimicrobial activity of the archaea EPs, we selected 80 peptides that were both sequentially diverse (<70% sequence similarity with each other) and top ranked by APEX 1.1 (Supplementary Data 2). We prioritized peptides with less than <70% sequence similarity to known AMP sequences for chemical synthesis and experimental validation (Extended Data Fig. 2a). In addition, when two mined sequences showed high sequence similarity, we retained only the peptide with the higher predicted antimicrobial activity (see ‘Archaeasin selection’ in Methods).
These archaeasins were tested against clinically relevant pathogens (Acinetobacter baumannii, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, Enterococcus faecalis and Enterococcus faecium) at a range of concentrations from 1 μmol l−1 to 64 μmol l−1. The results showed that 75 of the 80 EPs showed antimicrobial activity (MIC ≤ 64 μmol l−1) against at least 1 pathogenic strain (Fig. 2a), resulting in a hit rate of over 93%. Polymyxin B and levofloxacin were used as positive controls (Extended Data Fig. 4a). In addition, the Pearson correlation (r = 0.503) between predicted and experimentally validated MICs showed the predictive power of APEX 1.1 (Extended Data Fig. 4b). When comparing the Pearson and Spearman correlations between experimental and predicted MICs from the first version of APEX6 to APEX 1.1 (used to explore the archaeome) on the 80 archaeasins synthesized, we observed that APEX 1.1 significantly outperformed APEX (Supplementary Tables 3 and 4).
a, Heat map showing the antimicrobial activities (μmol l−1) of active antimicrobial agents from archaea against 11 clinically relevant pathogens, including Gram-negative (indicated by –) and Gram-positive (indicated by +) antibiotic-resistant strains (CRE, colistin-resistant Escherichia coli; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococci). Briefly, 105 bacterial cells were incubated with serially diluted EPs (0–64 μmol l−1) at 37 °C. Bacterial growth was assessed by measuring the optical density at 600 nm in a microplate reader at 1 day post-treatment. The MIC values presented in the heat map represent the mode of the replicates for each condition, and the antibiotics polymyxin B and levofloxacin were used as positive controls (Extended Data Fig. 4a). b−d, Ternary plots showing the percentage of secondary structure for each peptide (at 50 μmol l−1) in 3 different solvents: water (b), 60% TFE in water (c), and SDS (10 mmol l−1) in water (d). Secondary structure fractions were calculated using the BeStSel server18. fH and fβ stand for helical and β fractions, respectively. Red dots indicate active archaeasins and blue dots represent inactive peptides (Extended Data Fig. 5).
Disordered and β-rich secondary structure profiles of archaeasins
The secondary structure of short peptides is often dynamic, transitioning between disordered and ordered conformations at hydrophobic–hydrophilic interfaces. These structural transitions are critical in determining the antimicrobial and other biological functions of peptides. To assess the secondary structure of the synthesized archaeasins, we conducted circular dichroism experiments in various environments: water, sodium dodecyl sulfate (SDS) in water (10 mmol l−1), and a mixture of trifluoroethanol (TFE) in water (3:2, v/v). SDS micelles were chosen as a membrane-mimetic environment because of the lipid bilayer environment that is similar to biological bilayers15. The TFE–water mixture is known to induce α-helical structures by dehydrating the amide groups in the peptide backbone, thus favouring intramolecular hydrogen bonds that promote a helical conformation16,17. All archaeasins were tested at 50 μmol l−1 in the wavelength range of 190–260 nm (Extended Data Fig. 5). To determine secondary conformation fractions, we used the Beta Structure Selection (BeStSel) server18 (Fig. 2b–d). As expected, given that archaeasins are short sequences (<50 amino acid residues), all tested peptides were unstructured in water (Fig. 2b), with a slight tendency towards β-like structures (20% < fβ (β fraction)< 45%) in the other 2 analysed media, the helical-inducer TFE and water mixture (3:2, v/v; Fig. 2c) and SDS micelles (10 mmol l−1) in water (Fig. 2d). This behaviour is typical for short peptides showing antimicrobial activity19,20,21. Whereas EPs primarily adopt β-like structures3,8, archaeasins showed helical conformations in helical-inducing media and upon interaction with lipid bilayers.
Functional synergy and cooperative interactions among archaeasins
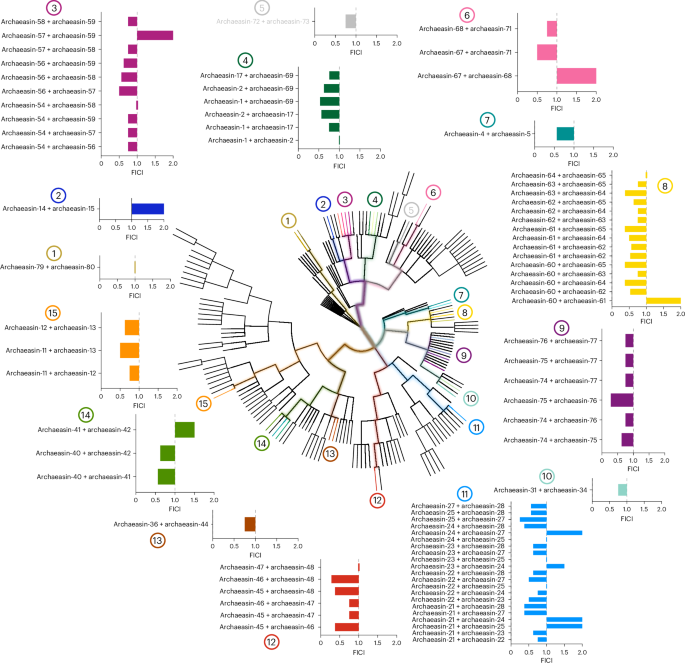
To explore whether molecules from the same archaeal strains or their closest relatives could synergize and potentiate each other’s antimicrobial activity against pathogens, we performed checkerboard assays. Checkerboard assays are a standard method used to evaluate the interaction between two antimicrobial agents by testing their combined effects over a range of concentrations. This approach allows for the determination of whether a combination exhibits synergy, additivity, indifference or antagonism, providing a quantitative assessment of potential cooperative activity22. These assays tested peptide concentrations ranging from 2× the MIC to concentrations up to 32× lower, under the same conditions as those used for the antimicrobial assays. We initially selected the bacterial strain A. baumannii American Type Culture Collection (ATCC) 19606, known for its high antibiotic resistance and significant role as an opportunistic nosocomial pathogen with substantial global mortality rates23. This strain was particularly susceptible to the archaeasins. We then selected peptides from strains closely related on the phylogenetic tree (pairwise distance ≤8), resulting in the testing of 79 pairs of archaeasins (Fig. 3).
The synergistic interactions between pairs of EPs from the same or closely related organisms (phylogenetic pairwise distance ≤8) that showed activity against A. baumannii ATCC 19606 were assessed using checkerboard assays. These assays involved twofold serial dilutions, ranging from 2× MIC to a 1:32 dilution. The histogram shows the FICI values obtained for each pair of EPs. A total of 79 pairs were evaluated. Low FICI values (≤0.5) indicate synergistic interactions, intermediate values (0.5 < FICI ≤ 1) indicate additive effects and higher values (1 < FICI ≤ 2) indicate indifferent interactions. Numbers 1–15 indicate where each pair or group of pairs originates within the archaeal phylogenetic tree.
Most of the combinations tested showed synergistic or additive interactions, as determined by the fractional inhibitory concentration index (FICI)24. The FICI is commonly classified as follows: FICI ≤ 0.5 indicates strong synergy, 0.5 < FICI ≤ 1 suggests an additive effect, 1 < FICI ≤ 2 implies no interaction (indifference) and FICI > 2 denotes antagonism between the compounds. Lower FICI values indicate a stronger interaction, where the combined effect of the peptides enhances antimicrobial efficacy beyond their individual activities. Synergistic interactions are particularly significant in antimicrobial research, as they allow for lower individual drug concentrations while maintaining or enhancing efficacy. This can reduce potential toxicity, slow resistance development and improve treatment outcomes. By leveraging synergy, it may be possible to develop combination therapies that enhance the effectiveness of existing antimicrobial agents. Notably, archaeasins from Methanocaldococcus species showed some of the lowest FICI values, ranging from 0.25 (archaeasin-25 and archaeasin-27) to 0.375 (archaeasin-21 and archaeasin-27, archaeasin-21 and archaeasin-28, and archaeasin-24 and archaeasin-28). Similarly, Methanothermobacter species compounds showed FICI values from 0.28 (archaeasin-46 and archaeasin-48) to 0.375 (archaeasin-45 and archaeasin-46, and archaeasin-46 and archaeasin-48). Thermococcus species had a FICI of 0.28 (archaeasin-75 and archaeasin-76), while compounds derived from Pyrococcus species had a FICI of 0.375 for combinations of archaeasin-60 and archaeasin-64, archaeasin-60 and archaeasin-65, archaeasin-61 and archaeasin-65, and archaeasin-63 and archaeasin-64.
Interestingly, our analysis revealed that certain archaeal lineages appear to be more prone to producing synergistic peptides. Peptides from hyperthermophilic archaea, particularly those from Methanocaldococcus and Thermococcus species, showed the most consistent synergistic interactions. This observation suggests that organisms adapted to extreme environments may have evolved antimicrobial strategies that rely on cooperative mechanisms, possibly to counteract competitive microbial communities. The tendency of peptides from these lineages to show synergy could be due to their structural adaptations, enhanced stability under extreme conditions or specific physico-chemical properties that facilitate cooperative activity. Further exploration of these evolutionary trends may provide deeper insights into the origins of antimicrobial synergy and guide the design of therapeutic peptide combinations.
These findings highlight the potential for archaeasin-based combinatorial therapies to provide alternatives for antimicrobial development, particularly in addressing multidrug-resistant infections.
Membrane-disruptive mode of action of archaeasins
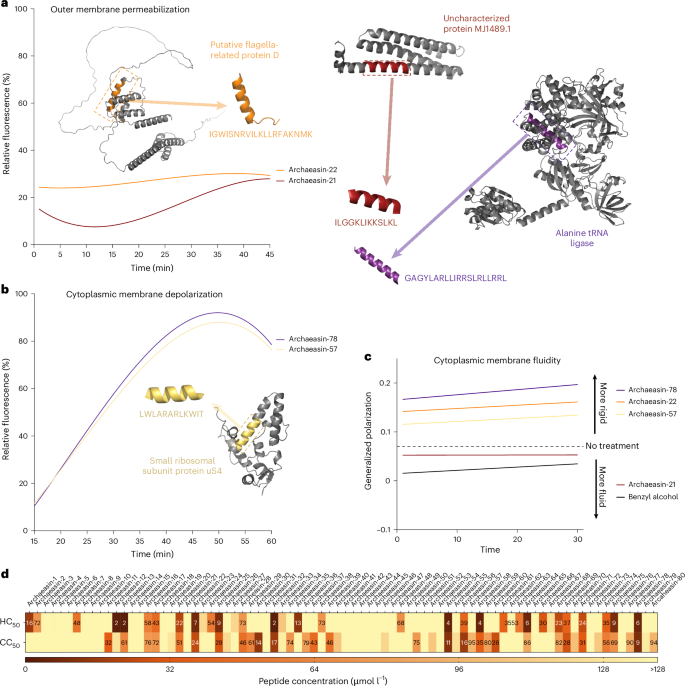
To understand how archaeasins exert their effect on bacterial cells, we conducted fluorescence assays to determine if their mechanism of action involves membrane targeting. First, we identified 70 antimicrobial hits among archaeasins effective against A. baumannii ATCC 19606 (Fig. 2a). We then assessed the ability of these peptides, at their MIC values, to permeabilize (Fig. 4a and Extended Data Fig. 6a) and depolarize (Fig. 4b and Extended Data Fig. 6b) bacterial outer and cytoplasmic membranes, respectively.
To assess whether archaea EPs act on bacterial membranes, all active peptides against A. baumannii ATCC 19606 were subjected to outer membrane permeabilization and cytoplasmic membrane depolarization assays. a,b, Here we show the two lead permeabilizer and depolarizer archaeasins (see Extended Data Fig. 6 for permeabilization and depolarization results of all archaeasins). The fluorescence probe NPN was used to assess membrane permeabilization (a) induced by the tested EPs (Extended Data Fig. 6a). The fluorescence probe DiSC3-5 was used to evaluate membrane depolarization (b) caused by archaeasins (Extended Data Fig. 6b). The shown values represent the relative fluorescence of both probes, with nonlinear fitting compared with the baseline of the untreated control (buffer + bacteria + fluorescence dye) and benchmarked against the antibiotics polymyxin B and levofloxacin. c, Laurdan generalized polarization over time in A. baumannii treated with archaeasins. Generalized polarization values were measured to assess changes in the lipid packing (membrane order) of the cytoplasmic membrane following treatment with the archaeasins that showed greater permeabilization of the outer membrane, archaeasin-21 and archaeasin-22, and greater depolarization of the cytoplasmic membrane, archaeasin-57, and archaeasin-78. Higher generalized polarization values indicate increased membrane rigidity, whereas lower values reflect increased membrane fluidity. Benzyl alcohol was used as a positive control for membrane fluidization, and untreated cells served as a negative control. Data represent a linear regression of the mean of 3 independent experiments over 30 min. d, Haemolytic and cytotoxic concentrations, against RBCs and HEK293T cells, respectively, leading to 50% cell lysis (HC50 and CC50, respectively) were determined by interpolating the dose-response data using a nonlinear regression curve. All experiments were performed in three independent replicates (Extended Data Fig. 7). The protein and peptide structures depicted in panels a and b were created with PyMOL Molecular Graphics System, v.3.0 (Schrödinger).
To evaluate the ability of archaeasins to permeabilize the outer membrane of Gram-negative bacteria, we used N-phenyl-1-naphthylamine (NPN) assays. NPN is a lipophilic dye that fluoresces in lipid-rich environments, such as bacterial outer membranes. Damage to the bacterial outer membrane allows NPN to penetrate, increasing fluorescence (Fig. 4a). Only archaeasin-21 (parent protein, uncharacterized protein MJ1489.1) and archaeasin-22 (parent protein, putative flagella-related protein D) from Methanocaldococcus jannaschii effectively permeabilized the bacterial outer membrane. Polymyxin B served as a positive control in these experiments4. Overall, archaeasins did not permeabilize the bacterial outer membrane to the extent observed for AMPs25,26 or other human- or animal-derived EPs4,6.
We then used 3,3′-dipropylthiadicarbocyanine iodide (DiSC3-5), a fluorophore that indicates cytoplasmic membrane depolarization. Disruption of the transmembrane potential causes the fluorophore to migrate to the extracellular space, resulting in increased fluorescence. Among the 70 peptides tested, 34 archaeasins significantly depolarized the cytoplasmic membrane more than the control group treated with polymyxin B4 (Fig. 4b). Archaeasin-78 (parent protein, alanine tRNA ligase) from Thermofilum pendens and archaeasin-57 (parent protein, small ribosomal subunit protein uS4) from Pyrobaculum arsenaticum were particularly effective depolarizers.
Laurdan generalized polarization assays further supported this membrane-targeting mechanism by revealing changes in the physical state of the A. baumannii cytoplasmic membrane upon archaeasin treatment (Fig. 4c). Laurdan fluorescence shifts reflect alterations in membrane lipid packing, where increased generalized polarization values indicate membrane rigidification. Consistent with the cytoplasmic membrane depolarization data, archaeasin-78 and archaeasin-57 caused pronounced increases in generalized polarization values over time, suggesting that these peptides not only disrupt membrane potential but also directly perturb membrane lipid organization. In contrast, archaeasin-21 and archaeasin-22, which were effective at permeabilizing the outer membrane, induced only modest or even decreasing generalized polarization shifts, indicating minimal impact on the cytoplasmic membrane’s physical state. These differences in Laurdan response correlate with each peptide’s structural features and probable depth of membrane insertion, reinforcing the conclusion that cytoplasmic membrane interaction, rather than outer membrane permeabilization, is the dominant antimicrobial mechanism for most archaeasins.
These findings suggest that archaeasins primarily exert their antimicrobial effects by depolarizing the cytoplasmic membrane, rather than permeabilizing the outer membrane. This suggests a mechanism akin to that of the recently reported small open-reading-frame-encoded peptides8 and unusual for conventional AMPs25,26 and EPs4, which typically target the outer membrane19.
Low toxicity of archaeasins against human cell lines
To assess the potential toxicity of the synthesized archaeasins, we exposed them to human red blood cells (RBCs), a common method for evaluating the toxicity of antimicrobial agents20,26,27. Of the 80 archaeasins tested, 25 (31.3%) showed moderate to low haemolytic activity within the explored concentration range, that is, their HC50 values (linear regression of the peptide concentration that leads to 50% RBC lysis) were ≤64 μmol l−1 (Fig. 4d and Extended Data Fig. 7). Most sequences active against bacterial pathogens at low MIC values did not display toxic effects at those concentrations (Extended Data Fig. 7). However, 7 archaeasins, specifically archaeasin-12, archaeasin-13, archaeasin-32, archaeasin-54, archaeasin-58, archaeasin-64 and archaeasin-78, did show haemolytic effects.
To further evaluate the safety profile of the archaeasins, we assessed their cytotoxic activity against human embryonic kidney (HEK293T) cells. The CC50 values, defined as the peptide concentration leading to 50% reduction in HEK293T cell viability, were determined for each of the 80 synthesized archaeasins (Fig. 4d). The cytotoxicity data are summarized in a heat map, indicating the concentration ranges at which cytotoxic effects were observed (Fig. 4d and Extended Data Fig. 7).
Of the 80 archaeasins tested, the majority displayed low cytotoxicity, with CC50 values exceeding 128 µmol l−1. Specifically, 26 archaeasins showed CC50 values higher than 128 µmol l−1, suggesting minimal cytotoxic effects within the tested concentration range. However, a subset of archaeasins showed low to moderate cytotoxicity. Notably, archaeasin-12, archaeasin-13, archaeasin-32, archaeasin-54, archaeasin-58, archaeasin-64 and archaeasin78, which also showed haemolytic activity, had CC50 values at or below 64 µmol l−1, indicating potential off-target toxicity.
Interestingly, most archaeasins with potent antibacterial activity (low MIC values) did not show significant cytotoxicity towards HEK293T cells at those concentrations. This selective activity highlights their potential as promising antimicrobial candidates with limited cytotoxic effects on human cells.
Anti-infective activity of archaeasins in preclinical animal models
To evaluate whether the lead archaeasins retained their antimicrobial potency in complex living systems, we tested them in two mouse models: a skin abscess model28,29,30 and a deep thigh infection model4,5 (Fig. 5a). In both models, we used A. baumannii, a pathogen responsible for infections in the blood, urinary tract, lungs, and topical wounds, and a major cause of mortality in hospitalized patients due to its antimicrobial resistance31. Three lead archaeasins showed potent activity against A. baumannii and no cytotoxicity (CC50 < 64 μmol l−1): archaeasin-2 (MIC value = 4 μmol l−1) from Aeropyrum pernix, archaeasin-17 (MIC value = 2 μmol l−1) from Ignicoccus hospitalis and archaeasin-73 (MIC value = 8 μmol l−1) from Sulfurisphaera tokodaii.
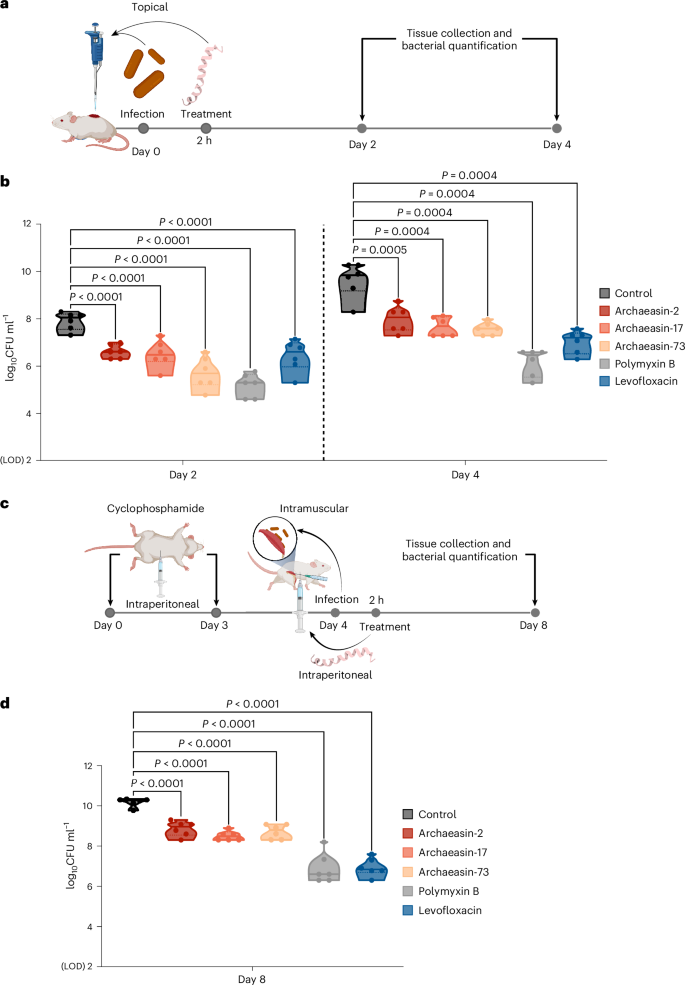
a, Schematic representation of the skin abscess mouse model used to assess the anti-infective activity of archaeasins (n = 6) against A. baumannii ATCC 19606. b, Archaeasin-2, archaeasin-17 and archaeasin-73, administered at their MIC in a single dose post-infection, inhibited the proliferation of the infection for up to 4 days after treatment compared with the untreated control group. Notably, archaeasin-73 reduced the infection in some mice, showing activity comparable to the control antibiotic, polymyxin B. c, Schematic of the neutropenic thigh infection mouse model, where archaeasins were administered intraperitoneally. Anti-infective activity against A. baumannii ATCC 19606 was evaluated 4 days after intraperitoneal peptide administration (n = 6). d, At 4 days after intraperitoneal injection (day 8 of the experiment), all archaeasins at their MIC showed a bacteriostatic effect, containing the A. baumannii ATCC 19606 infection, although their activity was less potent than that of polymyxin B and levofloxacin, compared with the untreated control group (Extended Data Fig. 8). The limit of detection (LOD) for the CFU quantification is log10CFU = 2. Statistical significance in panels b and d was determined using one-way analysis of variance followed by Dunnett’s test; P values are shown in the graphs. In the violin, the centre line represents the mean, the box limits the 1st and 3rd quartiles, and the whiskers (minimum and maximum) represent 1.5× the interquartile range. The solid line inside each box represents the mean value obtained for each group. Panels a and c created with BioRender.com.
In the skin abscess model, infection was established with a 20 μl bacterial load of 1.2 × 105 A. baumannii cells in phosphate buffer solution (PBS) applied to a wounded area of the skin (Fig. 5a). A single dose of each archaeasin at their respective MIC was administered to the infected area. Two days post-infection, all tested archaeasins showed significantly reduced bacterial counts by 1.5 to 2 orders of magnitude. Archaeasin-73, in particular, reduced the bacterial load by two orders of magnitude compared with the untreated control group. Its potency was comparable to that observed in the positive control group of mice treated with polymyxin B and was higher than that of the levofloxacin control group (Fig. 5b). Four days post-infection, all archaeasins and the two antibiotics, polymyxin B and levofloxacin, continued to prevent bacterial growth with similar efficacy. Polymyxin B reduced bacterial counts by four orders of magnitude compared with the untreated control group of mice, while all other treatment groups showed a two- to three-order magnitude decrease. These results are promising, as the archaeasins were administered only once after the abscess had been established, highlighting their anti-infective potential. Importantly, no significant changes in weight, used as a proxy for toxicity, were observed in our experiments (Extended Data Fig. 8a).
Next, we assessed the efficacy of the same lead archaeasins (archaeasin-2, archaeasin-17 and archaeasin-73) in a murine deep thigh infection model (Fig. 5c), which is widely used to assess the antibiotic potential of compounds. Mice were administered 2 rounds of cyclophosphamide treatment for immunosuppression before the intramuscular infection with 1 × 105 cells in 100 μl of A. baumannii. A single dose of each archaeasin (at their MIC) was delivered intraperitoneally (Fig. 5c). Four days post-treatment, the archaeasins were unable to prevent the growth of the infection, while the antibiotics polymyxin B and levofloxacin (positive controls) reduced the bacterial load by three orders of magnitude (Fig. 5d). Four days post-treatment, the bacterial counts remained stable for all peptide treatment conditions and the treatments with polymyxin B and levofloxacin, while the untreated control increased by two orders of magnitude. No significant changes in weight were observed, indicating that the archaeasins are non-toxic (Extended Data Fig. 8b). These in vivo results support the antibiotic properties of archaeasins under physiological conditions and provide a strong foundation for advancing their development as potential antimicrobial agents.
Source link