Researchers in the United Kingdom say they have successfully trialed what could become the world’s first gene therapy for Huntington’s disease – a fatal neurodegenerative disorder that is typically inherited.
While the results of the clinical trial are not yet formally published or peer reviewed, principal investigator and neuroscientist Ed Wild from University College London says the gene therapy, called AMT-130, “changes everything.”
The highest dose can apparently slow disease progression by as much as 75 percent over three years. It also led to a significant reduction in a biomarker of neurodegeneration, found in cerebrospinal fluid, which usually increases with disease progression.
Related: Huge Breakthrough as Experimental Drug Is First-Ever to Suppress Huntington’s Protein
“On the basis of these results, it seems likely AMT-130 will be the first licensed treatment to slow Huntington’s disease, which is truly world-changing stuff,” says Wild, who works at UCL’s Huntington’s Disease Center, the largest Huntington’s clinical group in Europe.
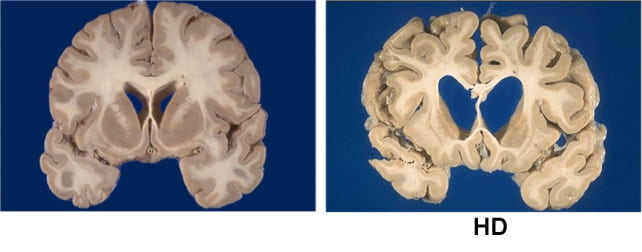
Huntington’s is caused by a single defect in the gene HTT, discovered in 1993, which gradually destroys the body’s ability to function. It does so by making a toxic version of the huntingtin protein, which attacks neurons in parts of the brain involved in voluntary movement, thinking, and behavior.
When visible symptoms take hold, typically in mid-adulthood, patients generally have just 10 to 30 years to live. A parent with Huntington’s has a 50 percent chance of passing the disease on to their children.
For roughly a decade, researchers at uniQure, the company behind the world’s first approved gene therapy, have been investigating if a similar method could work as a treatment for Huntington’s.
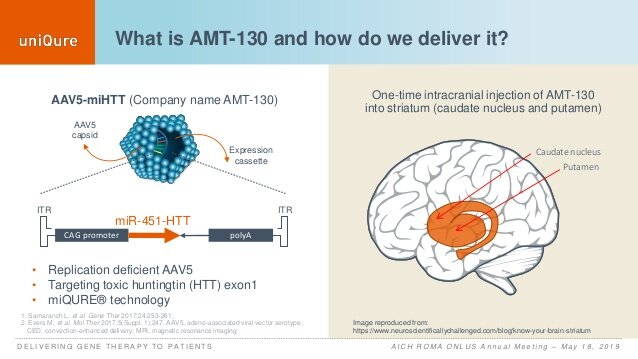
The company’s novel drug was tested by neurologists in the United Kingdom, as it involves major brain surgery. The idea is that when high-dose AMT-130 is injected into the brain, neurons take up the custom-made DNA permanently. The genetic information contains instructions that stop cells from making the mutant huntingtin protein.
The researchers expect the single dose to last a lifetime.
“Trial results come through in numbers and graphs, but behind each data point is an incredible patient who volunteered to undergo major neurosurgery to be treated with the first gene therapy we’ve ever tested in Huntington’s disease,” says Wild.
“That is an extraordinary act of bravery for the benefit of humanity.”
In the phase 1/2 clinical trial, 29 patients volunteered to be treated with AMT-130, with 17 receiving a high dose and 12 receiving a low dose. Researchers then followed up with a dozen patients from each group over the course of three years.
The high-dose group showed 75 percent less disease progression than participants who did not receive any AMT-130.
That’s not quite the “potentially curative results” the company was hoping to deliver, but it could still be a life-changing breakthrough and the first real treatment for the disease.
“My patients in the trial are stable over time in a way I’m not used to seeing in Huntington’s disease,” says Wild, “and one of them is my only medically retired Huntington’s disease patient who has been able to go back to work.”
The speed at which AMT-130 has come to clinical trials is impressive. In the span of roughly a decade, researchers at uniQure have taken promising preclinical data and replicated the results in animals and then humans.
The company is now conducting further clinical trials in the US and Europe.
The chief medical officer, Walid Abi-Saab, says uniQure is “eager to discuss the data with the Food and Drug Administration (FDA)… later this year, with the goal of submitting a Biologics License Application in the first quarter of 2026.”
The US FDA has already granted a Breakthrough Therapy designation and a Regenerative Medicine Advanced Therapy designation, both of which could speed up its approval process. After that, uniQure also plans to apply for approval in the UK and Europe.
The trial results will be presented at the HD Clinical Research Congress in October.
Source link


