Sign up for the Starts With a Bang newsletter
Travel the universe with Dr. Ethan Siegel as he answers the biggest questions of all.
Our Universe, as we observe it today, is vast and isolated, with enormous amounts of space between the stars, and with stars clustered into trillions of galaxies scattered across tens of billions of light-years. It’s also extraordinarily cold; aside from the starlight that heats up matter locally, there’s only a very low-energy background of radiation coming from the cosmos itself: a thermal bath of blackbody radiation at 2.725 K, or less than three degrees above absolute zero. But our current situation only came about because our Universe has been expanding, gravitating, and cooling for the past 13.8 billion years.
Early on, it was smaller, denser, more uniform, and also hotter, leading all the way back to the earliest moments of the hot Big Bang, and to temperatures that far exceeded even the stupendous energies achieved at the Large Hadron Collider. At some point in cosmic history — in between then and now — the Universe itself must have been a rather temperate place: right around what we consider “room temperature” today. Could that have provided the perfect conditions for liquid water, and perhaps life, to have existed even in the depths of space? That’s what Jimmy Lau wants to know, writing in to ask:
“Can there be a time in the universe where the uniform temperature is about 300K, where big batches of liquid water were just floating in space? If mixed with organic compounds, could that floating water be the origin of life?”
Normally, when we talk about the origin of life, we assume a set of conditions that are similar to what was found in the environment of early Earth. But that’s not the only possibility. What can we say about this early, even pre-planetary origin scenario for life in the cosmos?

Deep under the sea, around hydrothermal vents, where no sunlight reaches, life still thrives on Earth. How to create life from non-life is one of the great open questions in science today, but hydrothermal vents are one of the leading locations where the first metabolic processes, the precursor to living organisms, may have first arisen. If life can exist down there on Earth, perhaps any aqueous environment may support it, such as on a small, cold moon or even in interstellar space.
Credit: NOAA Office of Ocean Exploration and Research
When it comes to the origin of life, we have to remember that there are so many things we remain completely ignorant about. We know that Earth is inhabited, and that life has persisted here, in an unbroken chain of success, on our world for more than 3.5 billion years, and perhaps even longer. We know that it’s possible that life arose naturally on our world from non-living raw ingredients, but that it’s also plausible that life began elsewhere in the Universe and came to Earth via a process known as panspermia, before taking hold here and leading to all the organisms that have arisen since.
We know that all living things found here on Earth share certain traits:
- they have a metabolism,
- they have a wall or membrane separating their interiors from their outside environments,
- they use energy and respond to stimuli from their environments,
- and they have an information-containing “code” within them, in the form of nucleic acids, that encode the production of proteins and other molecules essential for carrying out their biological functions.
However, we don’t know if a cell-like structure is required for life, or if that’s simply what’s survived and thrived here on Earth. We don’t know where or how life began on Earth, or even whether “life on Earth” got its start on Earth.
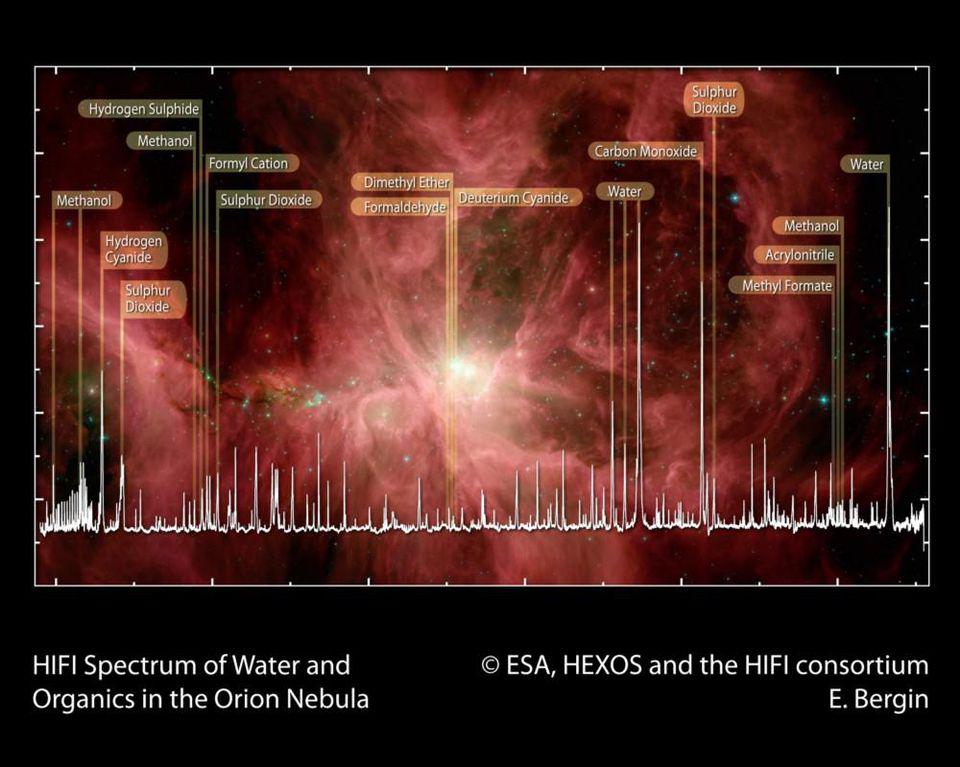
The raw ingredients that we believe are necessary for life, including a wide variety of carbon-based molecules, are found not only on Earth and other rocky bodies in our Solar System, but in interstellar space, such as in the Orion Nebula: the nearest large star-forming region to Earth.
It’s very dangerous to draw broader conclusions about life elsewhere in the Universe from a sample size of one, as Earth is the only world we know for certain is inhabited. Many of the things we know about life on Earth are often assumed to be true about life elsewhere, but we have no proof that these properties are universal. For example:
- all life on Earth relies on the presence of liquid water,
- all life on Earth leverages 20-22 amino acids, all of which are left-chiral,
- all life on Earth uses RNA and/or DNA to encode its genetic information,
- and all life on Earth can be genetically traced back to what we call LUCA, or the Last Universal Common Ancestor, suggesting a single origin for life, rather than multiple, independent origins.
We find life even in extreme environments here on Earth: at hydrothermal vents at the bottom of the ocean, within the interior craters of active volcanoes, deep underground in the interior of Earth itself, high up above the clouds in our atmosphere, and within ices that have been frozen for at least hundreds of thousands of years. There are very good reasons to think that other environments, even very different environments from any found here on Earth, may give rise to life as well, possibly relying on other forms of liquid than water, on other amino acids than those used on Earth, with peptide-based nucleic acids instead of DNA or RNA, and with a variety of possible origin stories.

In order for life to emerge within the Universe, the chemical precursor ingredients need to be delivered to an environment where life can arise, sustain itself, and thrive. This cannot happen until the elements required for life, including carbon, nitrogen, oxygen, and phosphorus, exist. None of them were created in the hot Big Bang.
However, one thing we can be certain of is this: even though it’s very likely, given what we know about how the Universe has evolved and grown up, that the emergence of life in the Universe was possible long before the formation of planet Earth, it wasn’t possible right from the start of things. Life, at least the kind of life that we know of, is chemical-based and relies on the interactions of atoms and molecules, which requires the existence of a variety of atoms, capable of bonding together to form a variety of structures. While we can imagine a very different set of molecules giving rise to life than are used in those processes here on Earth, we still require a certain set of raw ingredients — atoms — to consider the possible emergence of life at all.
However, this provides us with a very strong limitation on what could have arisen and when. The Big Bang itself didn’t provide a wide variety of atoms for us to work with as far as raw ingredients are concerned. Although protons, neutrons, and electrons were common early on, and there was a period of nuclear fusion early on (known as Big Bang Nucleosynthesis), the only raw ingredients we had to work with prior to the formation of stars were atoms whose nuclei were:
- bare protons, or “normal” hydrogen, representing 92% of atoms by number (and ~75% by mass),
- alpha particles, or helium-4 nuclei, representing ~8% of atoms by number,
- deuterium, or hydrogen-2 (with one proton and one neutron), representing ~0.001% of atoms by number,
- helium-3, a light isotope of helium, representing about ~0.0003% of atoms by number,
- lithium atoms (both Li-6 and Li-7), representing about ~0.0000001% of atoms,
- beryllium atoms (like Be-9, not Be-7, which is unstable and decays to lithium), representing 1-in-1012 atoms,
- and then tiny traces of carbon, nitrogen, and oxygen, representing 1-in-1014, 1-in-1017, and 1-in-1018 atoms, respectively.
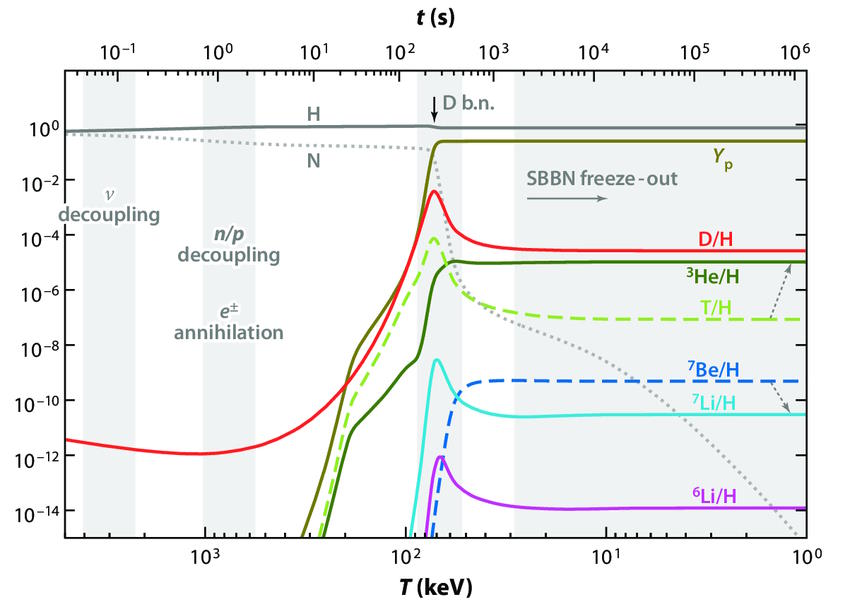
This plot shows the abundance of the light elements over time, as the Universe expands and cools during the various phases of Big Bang Nucleosynthesis. By the time the first stars form, the initial ratios of hydrogen, deuterium, helium-3, helium-4, and lithium-7 are all fixed by these early nuclear processes. Even smaller amounts of still-heavier elements, including carbon and oxygen, are produced, but not in any significant abundances.
Credit: M. Pospelov & J. Pradler, Annual Review of Nuclear and Particle Science, 2010
It is widely considered that carbon, nitrogen, oxygen, and hydrogen are indispensable elements for life, with phosphorus, sulfur, sodium, calcium, potassium, chlorine, magnesium, and other elements also normally treated as requirements. This is because if all you have are the simplest ingredients — which, in terms of any large abundance, are exclusively hydrogen and helium atoms prior to the formation of the first stars — the types of molecules you can make are restricted to being very simple. In fact, hydrogen and helium can only bind together into a few different combinations:
- monatomic hydrogen,
- monatomic helium,
- neutral hydrogen gas (H2),
- and helium hydride (HeH+),
and not much else.
This is important, because in order to engage in the type of chemical reactions that can lead to a metabolism, that can extract energy from the environment, and to create complex, differentiated structures (even simple molecular structures) that can conduct life processes, as well as the ability to encode information that can lead to reproduction or self-replication, a wider variety of molecules is required. That means we need heavier elements capable of more complex bonding possibilities than hydrogen and helium alone, and that means we need some way to create those elements.

The very first stars to form in the Universe were different than the stars today: metal-free, extremely massive, and nearly all destined for a supernova surrounded by a cocoon of gas. Heavy elements are produced inside the cores of these stars, including the elements necessary for the production of complex, organic molecules, and eventually, life.
Credit: NAOJ
In other words, to make the molecules we need for life, we need stars to produce those atoms. If we assume we need water, and water is made of both hydrogen (which is always abundant) and oxygen (which is only abundant once the first stars have ignited), then we again need stars to produce those atoms. So this gives us two big scientific questions to ask if we want to consider the possibility that life arose early on in cosmic history: back when the Universe was room temperature.
- At what epoch in cosmic history did we achieve those room temperature conditions?
- And how does that epoch compare to the earliest possible epoch in which stars could have potentially formed, lived, died, and enriched the space around them with the first heavy elements?
The nice thing about these questions is that they give us a method for comparing if life was possible back when the Universe was room temperature. If there were stars forming, living, and dying prior to the Universe becoming room temperature, there could have been not just stores of water, but potentially complex molecules existing alongside that water, at temperatures that are conducive to water existing in the liquid phase. While you might argue that we need a planet to have that water be under enough pressure to be in the liquid phase, a quick look at the phase diagram of water (below) shows that you don’t need very much pressure at all: about 1% of Earth’s atmospheric pressure will do, and temperatures can actually be below 300 K, all the way down to about 273 K at these low pressures (and down to ~251 K at extremely high pressures), while still allowing water to persist in its liquid phase.

At a variety of temperatures and pressures, water will take on a variety of states: solid, liquid, and gas. With high enough pressures, so long as your ice temperature remains above -8 F (-22 C), ice can melt from the Ih phase, the most common phase found on Earth, into the liquid phase.
As the Universe expands, it cools for a specific reason: because any photons that travel through that Universe have their wavelength get stretched as the Universe expands. For photons — particles of light — their energy is defined by their wavelength, and so as their wavelength stretches, they lose energy. A large system of particles with energy, even if those particles of photons, also has an associated temperature, and so lengthening those wavelengths corresponds to cooler, lower temperatures.
But the reverse of that is also true: if we imagine that the Universe’s clock can be run backward, instead of just forward, then that leftover radiation from the Big Bang that’s at 2.725 K today would have had shorter wavelengths, and higher temperatures, as a result. Because this corresponds to an epoch where the Universe was younger, smaller, more uniform (less clumpy), and also hotter, we can simply use what we know about the expanding Universe today to extrapolate back, and ask, “when was the Universe at any particular temperature?” Some interesting values for temperature (T), redshift (z), how great of a distance we need to look back to see that epoch in cosmic history (d), and time since the Big Bang (t), are as follows:
- T = 100 K, z = 35.7, d = 38.4 billion light-years, t = 76 million years,
- T = 251 K, z = 91, d = 41.5 billion light-years, t = 19 million years,
- T = 273 K, z = 99, d = 41.7 billion light-years, t = 17 million years, and
- T = 300 K, z = 109, d = 42.0 billion light-years, t = 14 million years.
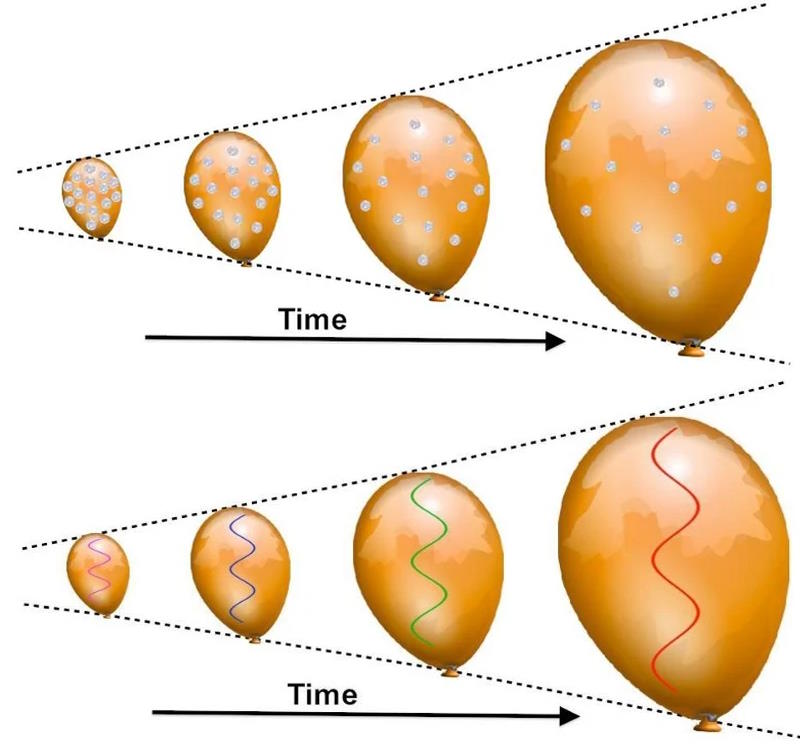
As a balloon inflates, any coins glued to its surface will appear to recede away from one another, with “more distant” coins receding more rapidly than the less distant ones. Any light will redshift, as its wavelength ‘stretches’ to longer values as the balloon’s fabric expands. This visualization solidly explains cosmological redshift within the context of the expanding Universe. If the Universe is expanding today, that implies a past where it was smaller, hotter, denser, and more uniform: leading to the picture of the hot Big Bang, and with higher temperatures in the past, the farther away we look.
Credit: E. Siegel/Beyond the Galaxy
For comparison, the earliest, most distant confirmed object in cosmic history is galaxy MoM-z14, whose light comes to us when the Universe is already much, much older and more evolved than any of these scenarios: when it had a temperature of T = 42 K, a redshift of z = 14.3, a distance of d = 33.8 billion light-years, and an age of 286 million years. The most distant candidate object ever spotted by JWST, which is heavily disputed and has neither been confirmed nor followed-up on spectroscopically, would (if real) come from when the Universe’s temperature was T = 70 K, at a redshift of z = 25, at a distance of d = 36.8 billion light-years, and a cosmic age of t = 128 million years.
In theory, we can ask when the very first stars of all form, and estimates vary wildly: from as late a time as t = 98 million years (and a temperature of T = 84 K to as early a time as t = 28 million years (and a temperature of T = 193 K). You can ask, “okay, but is it possible that we formed stars even earlier than the earliest models and simulations indicate?”
Perhaps it is possible, but such a scenario would require some sort of non-standard physics, beyond the standard types of structure formation that are associated with the actual temperature fluctuations that we see in the cosmic microwave background. Those temperature fluctuations correspond to density fluctuations, where it’s the overdense regions that gravitationally collapse, and have the potential to form stars, first.
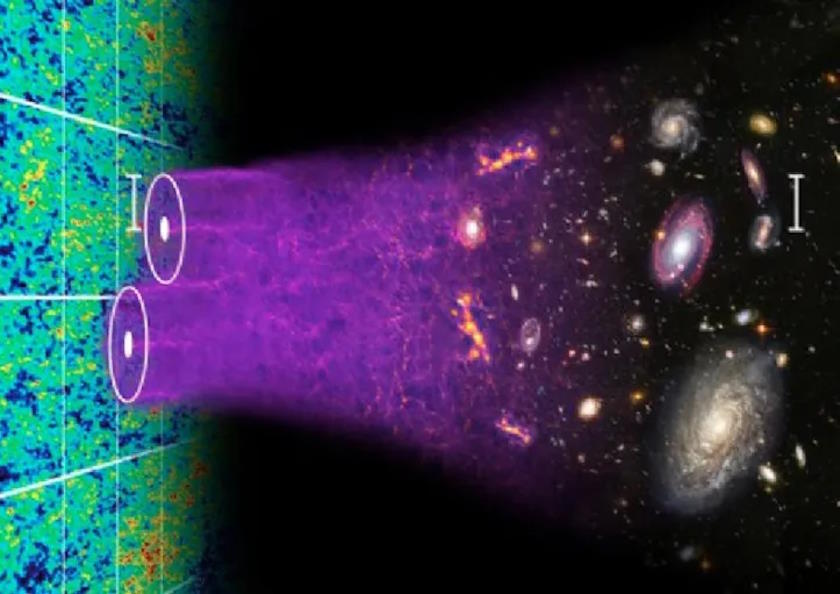
The temperature fluctuations that we see in the cosmic microwave background correspond to density fluctuations in the early Universe, with cold spots corresponding to overdensities. The “coldest” cold spots are the greatest initial overdensities, and collapse first to form stars, and later on, galaxies and galaxy clusters. The earliest stars, once they live-and-die, will enrich the interstellar medium with heavy elements: required ingredients to bring about the emergence of life.
Credit: Chris Blake & Sam Moorfield
The first stars, however, were likely:
- higher in mass,
- brighter,
- bluer,
- hotter,
- and shorter-lived,
than even the shortest-lived, most massive stars that form today. Whereas the most massive star ever spotted in our modern Universe caps out at ~260 solar masses, the stars that were theorized to form very early on may have been many hundreds, a thousand, or even several thousand solar masses. With such high masses, those stars could live, die, and enrich the Universe with heavy elements in as little as just one million years.
But that still requires that we form them incredibly early on. For demanding that we have those very first stars already be dead by the time the Universe is still at a temperature of T = 273 K or more, we require that the first stars form in only ~16 million years. That just doesn’t seem to be possible given what we know about the physics of the early Universe, and the conditions that were in place at the epoch that the cosmic microwave background (CMB) was emitted: at an age of t = 380,000 years. The CMB tells us how dense the densest regions of space were at that time, while the rules of general relativity and the physics of the expanding Universe enable us to calculate how long it takes for those overdensities to grow into regions that could potentially form stars. Put simply, the Universe doesn’t grow to be dense enough, fast enough, anywhere to have formed stars before it becomes too cold for water to exist in the liquid state.

At some point in cosmic history, the neutral matter that forms after the Big Bang contracts, with sufficient mass, to heat up and begin emitting visible light, and eventually, to ignite nuclear fusion in its core and begin producing heavy elements, including carbon, nitrogen, and oxygen. Simulations indicate that the very first stars should form when the Universe is between ~30 and 80 million years old, by which time the Universe is already much colder than “room temperature.”
Credit: NASA / WMAP Science Team
However, that will change when we’re no longer talking about being reliant on the leftover glow from the Big Bang — this thermal bath of photons that makes up today’s cosmic microwave background — to provide a source of heat and to determine the ambient temperature. The trigger for that stage is the collapse of gas clouds and the trapping of heat inside, which not only leads to the formation of stars, but explains where stars get the majority of their heat from. (Surprise, it’s not from nuclear fusion!) Once we transition from a state where the leftover glow of the Big Bang dominates the local temperature to the proximity to stars and sub-stellar objects determining the local temperature, at last we can have liquid water again.
And then, the thinking goes, if we can have liquid water, perhaps we can have life. It may yet be the case that life, forming under some exotic set of conditions, predates Earth-like, rocky planets. It’s plausible that life may not even need a planet at all to form, but could form elsewhere in space under the right conditions. But “the right conditions” weren’t in place back when the Universe was room temperature, between about 10 and 17 million years after the Big Bang. One of the necessary conditions for life is the presence of complex, heavy elements, and that requires stars to form, which in turn requires more time than that for the Universe we actually inhabit. Life may indeed have begun earlier than most scientists presently suspect, but not as early as when the Universe was at room temperature!
Send in your Ask Ethan questions to startswithabang at gmail dot com!
Sign up for the Starts With a Bang newsletter
Travel the universe with Dr. Ethan Siegel as he answers the biggest questions of all.
Source link
