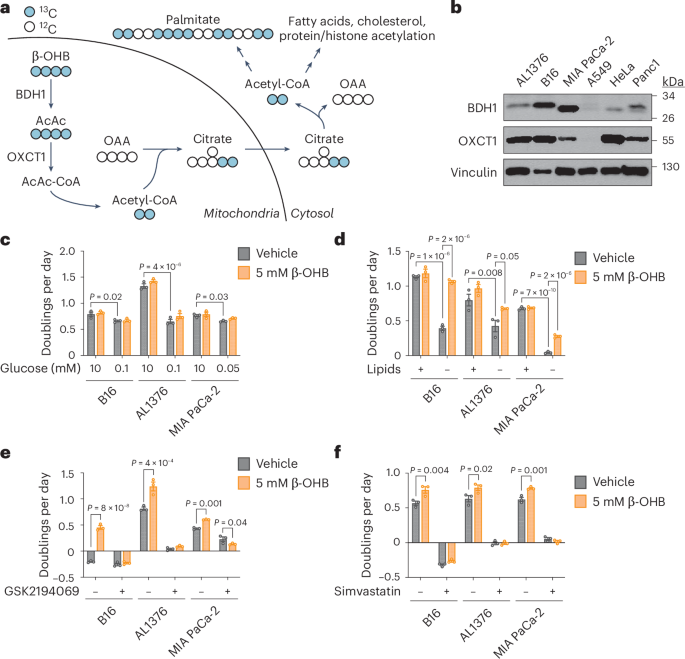
Under fasting conditions or low glycemic diets (such as a ketogenic diet or calorie restriction) that decrease blood glucose levels, ketogenesis occurs predominantly in the liver to produce ketone bodies. Ketone body oxidation then contributes to energy metabolism for extrahepatic tissues, thereby sparing glucose1. We and others recently demonstrated that some cancer cells can also metabolize β-hydroxybutyrate (β-OHB)2,3,4. β-OHB oxidation occurs in the mitochondria, where β-OHB dehydrogenase 1 (BDH1), 3-oxoacid CoA-transferase 1 (OXCT1) and a mitochondrial thiolase convert β-OHB into acetyl-CoA, which subsequently enters the tricarboxylic acid (TCA) cycle (Fig. 1a). We assessed BDH1 and OXCT1 protein levels in a panel of cancer cell lines and found three lines that strongly express both enzymes: AL1376, a pancreatic ductal adenocarcinoma (PDAC) cell line derived from the LSL-KrasG12D/+; Trp53fl/fl; Pdx1-Cre mouse PDAC model2,5; B16, a mouse-derived melanoma cell line; and MIA PaCa-2, a human PDAC cell line (Fig. 1b). Although β-OHB is an alternative to glucose for fuelling cellular energy production, we found that β-OHB could not rescue the proliferation defect of glucose-starved cells (Fig. 1c). We then noted that β-OHB is also a source of cytosolic acetyl-CoA. After β-OHB-derived acetyl-CoA is incorporated into citrate in the mitochondria, citrate can be exported into the cytosol and be used to generate cytosolic acetyl-CoA. Cytosolic acetyl-CoA has several downstream fates, including the synthesis of fatty acids, cholesterol, and protein and histone acetylation (Fig. 1a). To test the importance of cytosolic acetyl-CoA production from β-OHB, we took advantage of the observation that fatty acid synthesis is required for cancer cell proliferation when extracellular lipid levels are limiting6, and we asked whether β-OHB can rescue the proliferation defect of cells cultured in lipid-depleted media. Indeed, β-OHB promoted cell proliferation in lipid-depleted media across all three cell lines (Fig. 1d). In A549, HeLa and Panc1 cells that had lower BDH1 and/or OXCT1 expression (Fig. 1b), β-OHB did not exhibit this rescue (Extended Data Fig. 1a). Lipid-depleted media lacks both fatty acids and cholesterol, and consistently, the fatty acid synthase (FASN) inhibitor GSK2194069 and the cholesterol synthesis inhibitor simvastatin prevented β-OHB from rescuing the proliferation of lipid-starved cells (Fig. 1e,f). These data suggest that cytosolic acetyl-CoA is an important downstream fate of β-OHB.
a, Schematic of β-OHB metabolism and 13C labelling derived from [U-13C]-β-OHB. AcAc, acetoacetate; OAA, oxaloacetate. b, Immunoblot for BDH1, OXCT1 and vinculin in the indicated cancer cell lines. c, Proliferation rates of the indicated cancer cell lines grown in high or low glucose conditions, with or without 5 mM β-OHB. d, Proliferation rates of the indicated cancer cell lines grown in lipid-replete versus lipid-depleted culture media, with or without 5 mM β-OHB. e,f, Proliferation rates of the indicated cancer cell lines grown in lipid-depleted media, with or without 5 mM β-OHB and 0.3 µM of the FASN inhibitor GSK2194069 (e) or 25 µM of the cholesterol synthesis inhibitor simvastatin (f). Data are presented as means; error bars, s.e.m.; n = 3 biologically independent replicates. Comparisons were made using a two-way ANOVA (c–f).
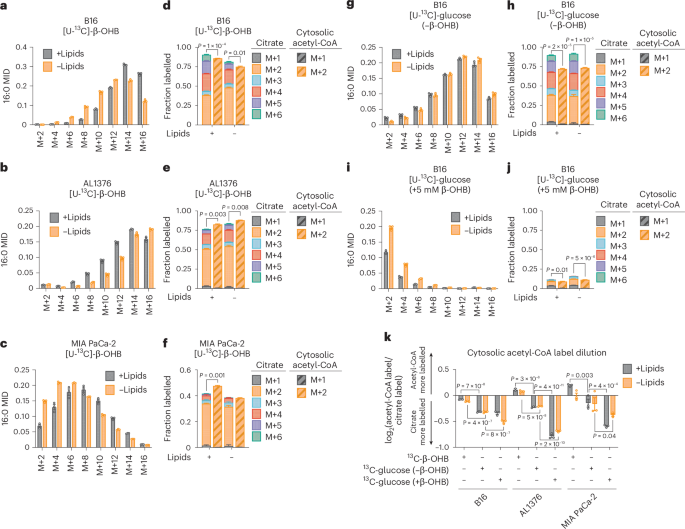
To examine the extent to which β-OHB contributes to the cytosolic acetyl-CoA pool, we took advantage of the concept that the labelling pattern of fatty acids such as palmitate by a stable isotope-labelled nutrient precursor directly reflects the fraction of cytosolic acetyl-CoA that is labelled by that precursor7. Labelling of palmitate by a stable isotope-labelled nutrient that fully labels the acetyl group in cytosolic acetyl-CoA results in even-numbered isotopomers (M+2, M+4,…M+16) because FASN synthesizes palmitate two carbons at a time using acetyl-CoA-derived malonyl-CoA. Importantly, the abundances of these isotopomers follow a binomial distribution, and at steady-state labelling, this mass isotopomer distribution (MID) reflects the fraction of cytosolic acetyl-CoA that is labelled. The further the MID is shifted to the right towards highly labelled isotopomers, the more cytosolic acetyl-CoA is labelled, and vice versa. Using this principle, a mathematical approach known as isotopomer spectral analysis (ISA) can use the binomial MID of palmitate to calculate the fraction of cytosolic acetyl-CoA that is labelled7,8.
We first confirmed that β-OHB is used to synthesize fatty acids by labelling B16, AL1376 and MIA PaCa-2 cells with 5 mM [U-13C]-β-OHB for 24 h and assessing fatty acid labelling. β-OHB labelled both saturated fatty acids, such as palmitate (16:0), and monounsaturated fatty acids, such as palmitoleate (16:1(n-7)) and oleate (18:1(n-9)), and this labelling was impaired by the FASN inhibitor GSK2194069 (Extended Data Fig. 1b–d). Moreover, fatty acid labelling was greater in cells grown in lipid-depleted media (Extended Data Fig. 1b–d), reflective of increased fatty acid synthesis. Next, achieving steady-state labelling is important for calculating cytosolic acetyl-CoA labelling by ISA. Given that fatty acids turn over slowly, 2–3 days of labelling are typically needed to approach steady-state labelling7. Indeed, we found that the palmitate MID continued shifting to the right between 24 h and 48 h of labelling (Extended Data Fig. 1e–g). Therefore, we conducted all subsequent stable isotope labelling experiments for 48 h.
In B16, AL1376 and MIA PaCa-2 cells, we detected substantial labelling of palmitate by 5 mM [U-13C]-β-OHB in both lipid-replete and lipid-depleted media (Fig. 2a–c). Notably, at 48 h of labelling, lipid limitation did not robustly increase 13C enrichment into palmitate (that is, an upward shift in the MID) (Fig. 2a–c), as was observed when labelling for 24 h (Extended Data Fig. 1b). Given that steady-state labelling reveals the contribution of β-OHB to the palmitate pool, rather than the flux of palmitate synthesis, the lack of an upward shift in the palmitate MID suggests that in both lipid-replete and lipid-depleted conditions, the degree to which β-OHB is used as a fuel for palmitate (versus other carbon sources) is similar. As expected, we also observed 13C labelling in citrate, the precursor of cytosolic acetyl-CoA (Fig. 2d–f). Using ISA, we then calculated 13C enrichment in cytosolic acetyl-CoA and found that it was highly labelled by β-OHB. Strangely, we noticed that in some cases, such as for AL1376 and MIA PaCa-2 cells, cytosolic acetyl-CoA was labelled to a greater extent than citrate (Fig. 2e,f). This was surprising because labelling of downstream metabolites typically becomes more diluted, rather than enriched, compared to the labelling of their upstream precursors.
a–c, Palmitate (16:0) MID for B16 (a), AL1376 (b) and MIA PaCa-2 (c) cells labelled with 5 mM [U-13C]-β-OHB for 48 h in lipid-replete versus lipid-depleted culture media. d–f, Citrate MID (solid bars) and cytosolic acetyl-CoA MID (dashed bars) for B16 (d), AL1376 (e) and MIA PaCa-2 (f) cells labelled with 5 mM [U-13C]-β-OHB for 48 h in lipid-replete versus lipid-depleted culture media. g–j, 16:0 MID (g,i) and citrate and cytosolic acetyl-CoA MID (h,j) for B16 cells labelled with 10 mM [U-13C]-glucose for 48 h in lipid-replete versus lipid-depleted culture media, either without unlabelled β-OHB (g,h) or with 5 mM unlabelled β-OHB (i,j). k, Cytosolic acetyl-CoA label dilution from citrate, as calculated by the log2(fold change) of the total fraction of cytosolic acetyl-CoA labelled versus the total fraction of citrate labelled, in B16, AL1376 and MIA PaCa-2 cells under the indicated tracing conditions. Data are presented as means; error bars, s.e.m.; n = 3 biologically independent replicates. Comparisons were made using a two-tailed Student’s t-test (d,e,f,h,j) or a two-way ANOVA (k).
As a comparison, we labelled cells with 10 mM [U-13C]-glucose for 48 h and found that in this case, cytosolic acetyl-CoA labelling was always diluted relative to citrate labelling (Fig. 2g,h and Extended Data Fig. 2a–d). This suggests that higher labelling of cytosolic acetyl-CoA relative to citrate is unique to β-OHB and that β-OHB may somehow be more efficiently used to synthesize cytosolic acetyl-CoA compared to glucose. Consistently, adding 5 mM unlabelled β-OHB strongly suppressed 10 mM [U-13C]-glucose labelling of palmitate, citrate and cytosolic acetyl-CoA (Fig. 2i,j and Extended Data Fig. 2e–h). Given that 5 mM β-OHB is on the upper end of physiological β-OHB concentrations, it may displace [U-13C]-glucose labelling through mass action. In support of this idea, the β-OHB uptake rate by B16 cells was reduced when extracellular β-OHB was lowered from 5 mM to 1 mM, which is more physiological2,9 (Extended Data Fig. 2i). The 1 mM β-OHB still substantially displaced [U-13C]-glucose labelling of palmitate, citrate and cytosolic acetyl-CoA, but to a lesser extent than 5 mM β-OHB (Extended Data Fig. 2j,k, compare to Fig. 2g–j). Interestingly, we note that 1 mM β-OHB had a larger effect at displacing glucose labelling of cytosolic acetyl-CoA compared to citrate; citrate labelling was reduced by ~15% (from ~90% to ~75%), whereas cytosolic acetyl-CoA labelling was reduced by ~35% (from ~70% to ~35%) (compare Extended Data Fig. 2k to Fig. 2h). These data demonstrate that although the extent to which β-OHB is used for cytosolic acetyl-CoA synthesis depends on its extracellular availability, β-OHB is a major source of cytosolic acetyl-CoA even when glucose is available in excess.
To quantify the degree to which 13C labelling was diluted versus enriched in cytosolic acetyl-CoA relative to citrate, we calculated the fold change in labelled cytosolic acetyl-CoA compared to labelled citrate (Fig. 2k). This confirmed that [U-13C]-β-OHB highly labelled cytosolic acetyl-CoA, in some cases more than it labelled citrate, whereas [U-13C]-glucose labelling of cytosolic acetyl-CoA was always diluted compared to citrate and was further diluted by the presence of unlabelled β-OHB. Finally, we noted that cytosolic acetyl-CoA is also used for fatty acid elongation, including the generation of longer-chain polyunsaturated fatty acids (PUFAs) such as 20:3(n-6), 20:4(n-6), 22:4(n-6) and 22:6(n-3). Indeed, both [U-13C]-β-OHB and [U-13C]-glucose labelled these PUFAs, and unlabelled β-OHB again suppressed PUFA labelling by [U-13C]-glucose (Extended Data Fig. 2l–n).
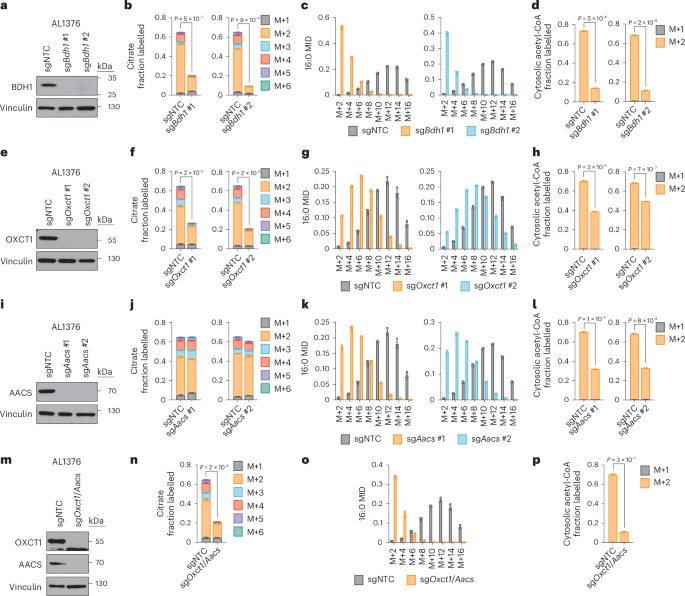
Based on these results, we reasoned that higher labelling of cytosolic acetyl-CoA relative to citrate can only occur if β-OHB is converted to cytosolic acetyl-CoA through a citrate-independent route. To test this idea, we used CRISPR–Cas9 to knock out Bdh1 or Oxct1 in AL1376 and B16 cells using two independent single guide RNAs (sgRNAs) (Fig. 3a,e and Extended Data Fig. 3a,e). We then labelled these cells with [U-13C]-β-OHB in lipid-depleted media. In Bdh1 knockout cells, citrate labelling was markedly reduced, as expected (Fig. 3b and Extended Data Fig. 3b). Bdh1 loss also shifted the palmitate MID almost completely to the left (Fig. 3c and Extended Data Fig. 3c) and thus markedly reduced cytosolic acetyl-CoA labelling (Fig. 3d and Extended Data Fig. 3d). Similar labelling patterns were observed in cells labelled in lipid-replete media (Extended Data Figs. 4a–c and 5a–c). Finally, Bdh1 loss also suppressed labelling of the omega-6 PUFAs 20:3(n-6), 20:4(n-6) and 22:4(n-6) (Extended Data Fig. 6a,c,e,f). Interestingly, residual labelling of citrate, cytosolic acetyl-CoA, palmitate and PUFAs in Bdh1 knockout cells suggests that there may be additional enzyme(s) with β-OHB dehydrogenase activity. One possibility is BDH2, although it is uncertain whether BDH2 can convert β-OHB to acetoacetate at physiological β-OHB concentrations because its Michaelis–Menten constant for β-OHB is ~10 mM (ref. 10).
a, Immunoblot for BDH1 and vinculin in the indicated AL1376 knockout lines. NTC, non-targeting control. b–d, Citrate MID (b), palmitate (16:0) MID (c) and cytosolic acetyl-CoA MID (d) in AL1376 sgNTC, sgBdh1 #1 and sgBdh1 #2 cells labelled with 5 mM [U-13C]-β-OHB for 48 h in lipid-depleted culture media. e, Immunoblot for OXCT1 and vinculin in the indicated AL1376 knockout lines. f–h, Citrate MID (f), 16:0 MID (g) and cytosolic acetyl-CoA MID (h) in AL1376 sgNTC, sgOxct1 #1 and sgOxct1 #2 cells labelled with 5 mM [U-13C]-β-OHB for 48 h in lipid-depleted culture media. i, Immunoblot for AACS and vinculin in the indicated AL1376 knockout lines. j–l, Citrate MID (j), 16:0 MID (k) and cytosolic acetyl-CoA MID (l) in AL1376 sgNTC, sgAacs #1 and sgAacs #2 cells labelled with 5 mM [U-13C]-β-OHB for 48 h in lipid-depleted culture media. m, Immunoblot for OXCT1, AACS and vinculin in the indicated AL1376 knockout lines. n–p, Citrate MID (n), 16:0 MID (o) and cytosolic acetyl-CoA MID (p) in AL1376 sgNTC and sgOxct1/Aacs cells labelled with 5 mM [U-13C]-β-OHB for 48 h in lipid-depleted culture media. Data are presented as means; error bars, s.e.m; n = 3 biologically independent replicates. Comparisons were made using a two-tailed Student’s t-test (b,d,f,h,j,l,n,p).
In Oxct1 knockout cells, labelling of citrate from [U-13C]-β-OHB was diminished to a similar extent as in Bdh1 knockout cells, as expected (Fig. 3f and Extended Data Fig. 3f). However, compared to Bdh1 loss, Oxct1 loss induced a weaker shift to the left in the palmitate MID (Fig. 3g and Extended Data Fig. 3g; compare to Fig. 3c and Extended Data Fig. 3c) and therefore did not reduce cytosolic acetyl-CoA labelling to the same extent (Fig. 3h and Extended Data Fig. 3h; compare to Fig. 3d and Extended Data Fig. 3d). Cytosolic acetyl-CoA was also labelled to a greater extent than citrate in Oxct1 knockout cells (compare Fig. 3h with Fig. 3f; Extended Data Fig. 3h with Extended Data Fig. 3f). Similar labelling patterns were observed in cells labelled in lipid-replete media (Extended Data Figs. 4d–f and 5d–f). Finally, Oxct1 loss suppressed PUFA labelling, but not to the same extent as Bdh1 loss (Extended Data Fig. 6b,c,e,f). These results confirm that β-OHB can bypass OXCT1-dependent mitochondrial citrate production to generate cytosolic acetyl-CoA.
We reasoned that mitochondrial acetoacetate (the substrate for OXCT1) is probably transported into the cytosol for cytosolic acetyl-CoA production. Acetoacetyl-CoA synthetase (AACS) is a cytosolic enzyme that produces acetoacetyl-CoA from acetoacetate, which can then be converted to cytosolic acetyl-CoA by acetoacetyl-CoA thiolases11. AACS is highly expressed in kidney, heart, brain, adipose tissue and osteoclasts12,13,14, and tracer studies with 14C-labelled β-OHB have demonstrated cytosolic activation of acetoacetate and its contribution to fatty acid and cholesterol synthesis in rat livers, brain, spinal cord, skin, adipose tissue and lactating mammary glands15,16,17,18,19,20. We therefore asked whether AACS also contributes to cytosolic acetyl-CoA synthesis from β-OHB in cancer cells. We knocked out Aacs (Fig. 3i and Extended Data Fig. 3i) and showed that citrate labelling from [U-13C]-β-OHB was minimally affected by Aacs loss, confirming that mitochondrial β-OHB entry into the TCA cycle remained intact (Fig. 3j and Extended Data Fig. 3j). However, Aacs loss shifted the palmitate MID to the left (Fig. 3k and Extended Data Fig. 3k), thus reducing cytosolic acetyl-CoA labelling (Fig. 3l and Extended Data Fig. 3l). Similar labelling patterns were observed in cells labelled in lipid-replete media (Extended Data Figs. 4g–i and 5g–i). Finally, Aacs loss also partially impaired PUFA labelling (Extended Data Fig. 6b,c,e,f). These results confirm that β-OHB can contribute to cytosolic acetyl-CoA through a citrate-independent route that requires AACS.
We next knocked out both Oxct1 and Aacs (Fig. 3m and Extended Data Fig. 3m), which shifted the palmitate MID almost completely to the left and reduced labelling of citrate, cytosolic acetyl-CoA and PUFAs by [U-13C]-β-OHB to the same extent as Bdh1 loss (Fig. 3n–p and Extended Data Figs. 3n–p, 4j–l, 5j–l and 6d,g). Finally, we also re-expressed Bdh1, Oxct1 and Aacs in their respective AL1376 knockout cells (Extended Data Fig. 7a,d,g). Re-expression of Bdh1 and Oxct1 restored β-OHB labelling of citrate (Extended Data Fig. 7b,e) and cytosolic acetyl-CoA (Extended Data Fig. 7c,f). Aacs re-expression did not increase citrate labelling, but did rescue cytosolic acetyl-CoA labelling (Extended Data Fig. 7h,i). Collectively, these results confirm that both the OXCT1-dependent and the alternative AACS-dependent routes contribute to cytosolic acetyl-CoA production from β-OHB.
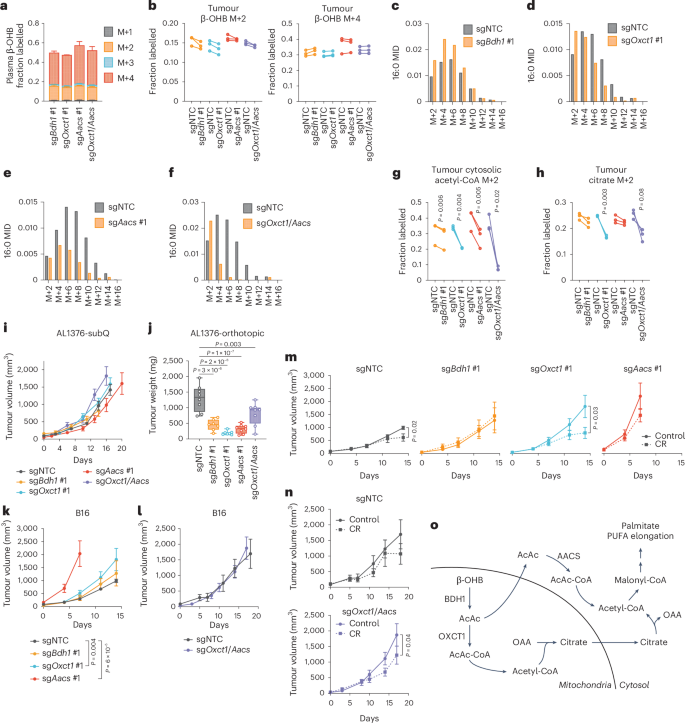
Next, we asked whether the AACS-dependent route also occurs in tumours in vivo. Slow lipid turnover makes it challenging to observe fatty acid labelling in in vivo stable isotope labelling experiments. We modified an established infusion protocol21 in which mice bearing AL1376 or B16 tumours were fasted for 16 h and then infused with [U-13C]-β-OHB through a tail vein catheter with a priming bolus of 478 mg kg−1 for 1 min, followed by a constant infusion at 9.75 mg kg−1 min−1 for 6.5 h. Under these parameters, the concentration of labelled β-OHB in plasma reached supraphysiological levels of >10 mM (Extended Data Fig. 8a), but even with these high concentrations, we only achieved substantial labelling of palmitate to observe its MID in B16, but not AL1376, tumours (Extended Data Fig. 8b). Therefore, we used the B16 model to ask whether the AACS-dependent route operates in vivo.
C57BL/6J mice were subcutaneously implanted with control B16 single guide non-targeting control (sgNTC) cells on one flank and either B16 sgBdh1 #1, sgOxct1 #1, sgAacs #1 or sgOxct1/Aacs cells on the other flank. After tumours formed, mice were infused with [U-13C]-β-OHB, resulting in ~50% 13C label enrichment in plasma β-OHB (Fig. 4a). Although [M+4] β-OHB was the predominant isotopomer in the plasma, a smaller fraction was [M+2]-labelled (Fig. 4a). This could potentially result from a ketogenic tissue breaking down [M+4] β-OHB into [M+2] acetyl-CoA, which was then combined with unlabelled acetyl-CoA to generate [M+2] β-OHB that was released into circulation. This highlights a limitation of in vivo tumour tracing studies, in which other tissues metabolize the infused labelled nutrient and transform it into other labelled metabolites that subsequently are available to tumours. Nevertheless, palmitate was minimally labelled in the plasma (Extended Data Fig. 8c), suggesting that any palmitate labelling in tumours probably resulted from fatty acid synthesis by tumour cells.
C57BL/6J mice bearing a B16 sgNTC tumour on one flank and a knockout tumour on the other flank were infused with [U-13C]-β-OHB for 6.5 h. a, Plasma β-OHB labelling in mice bearing the indicated knockout tumours. n = 3 mice for each knockout tumour. b, [M+2] and [M+4] fractional labelling of β-OHB in the indicated sgNTC versus knockout tumours. Data are paired between sgNTC and knockout tumours from the same mouse. n = 3 biological replicates for each knockout tumour. c–f, Representative palmitate (16:0) MIDs from n = 1 biological replicate of a mouse bearing an sgNTC tumour versus an sgBdh1 #1 tumour (c), sgOxct1 #1 tumour (d), sgAacs #1 tumour (e) and sgOxct1/Aacs tumour (f). See Extended Data Fig. 8 for additional biological replicates. g,h, [M+2] fractional labelling of cytosolic acetyl-CoA (g) and citrate (h) in the indicated sgNTC versus knockout tumours. Data are paired between sgNTC and knockout tumours from the same mouse. n = 3 biological replicates for each knockout tumour. i, Tumour volumes of the indicated subcutaneous AL1376 tumours in C57BL/6J male mice. sgNTC n = 6 mice, sgBdh1 #1 n = 4 mice, sgOxct1 #1 n = 6 mice, sgAacs #1 n = 4 mice and sgOxct1/Aacs n = 5 mice. j, Endpoint tumour weights of the indicated AL1376 tumours implanted orthotopically in the pancreas in C57BL/6J male mice. sgNTC n = 8 mice, sgBdh1 #1 n = 7 mice, sgOxct1 #1 n = 7 mice, sgAacs #1 n = 8 mice and sgOxct1/Aacs n = 8 mice. k,l, Tumour volumes of the indicated subcutaneous B16 tumours in C57BL/6J male mice. sgNTC n = 6 mice, sgBdh1 #1 n = 6 mice, sgOxct1 #1 n = 5 mice, sgAacs #1 n = 6 mice (k) and sgNTC n = 3 mice and sgOxct1/Aacs n = 4 mice (l). m,n, Tumour volumes of the indicated subcutaneous B16 tumours in C57BL/6J male mice exposed to a control or calorie-restricted diet. sgNTC control n = 6 mice, CR n = 6 mice; sgBdh1 #1 control n = 6 mice, CR n = 6 mice; sgOxct1 #1 control n = 5 mice, CR n = 5 mice; sgAacs #1 control n = 6 mice, CR n = 6 mice (m). sgNTC control n = 3 mice, CR n = 4 mice; sgOxct1/Aacs control n = 4 mice, CR n = 4 mice (n). o, Schematic of β-OHB contribution to cytosolic acetyl-CoA synthesis through both a mitochondrial citrate-dependent route through OXCT1 and a citrate-independent route through AACS. Data are presented as means; error bars, s.e.m (a,i,k–n) or as box-and-whisker plots displaying median, interquartile range (boxes) and minima and maxima (whiskers) (j). Comparisons were made using a two-tailed paired t-test (g,h), a one-way ANOVA (j) or a two-way ANOVA (i,k–n).
In tumours, we observed both [M+4] and [M+2] β-OHB labelling for a combined enrichment of ~50% (Fig. 4b). Within each mouse, β-OHB labelling was similar between the sgNTC tumour on one flank versus the knockout tumour on the other flank, enabling direct comparison of labelling patterns in metabolites downstream of β-OHB between the two tumours (Fig. 4b). Surprisingly, Bdh1 loss did not cause a robust shift to the left in the palmitate MID compared to sgNTC tumours (Fig. 4c and Extended Data Fig. 8d), and [M+2] cytosolic acetyl-CoA and [M+2] citrate labelling were minimally reduced in sgBdh1 tumours (Fig. 4g,h). One possible explanation for this may be that [U-13C]-β-OHB could also label circulating acetoacetate, which can bypass BDH1 in tumours to synthesize cytosolic acetyl-CoA through OXCT1 or AACS. Alternatively, another enzyme with β-OHB dehydrogenase activity, such as BDH2, may compensate for BDH1 loss. By contrast, we observed a stronger shift to the left in the palmitate MID in sgOxct1 and sgAacs tumours, and therefore a stronger decrease in cytosolic acetyl-CoA labelling (Fig. 4d,e,g and Extended Data Fig. 8e,f). As expected, Oxct1 loss, but not Aacs loss, decreased citrate labelling (Fig. 4h). Finally, loss of both Oxct1 and Aacs caused the strongest shift to the left in the palmitate MID (Fig. 4f and Extended Data Fig. 8g), an almost complete loss in cytosolic acetyl-CoA labelling (Fig. 4g) and decreased citrate labelling (Fig. 4h). These results demonstrate that β-OHB-derived cytosolic acetyl-CoA in tumours in vivo is also synthesized through both the mitochondrial citrate-dependent OXCT1 route and the cytosolic citrate-independent AACS route.
Interestingly, we found distinct effects of each gene knockout on PUFA labelling. Labelling of 20:3(n-6) was reduced in all knockout tumours, particularly in sgAacs and sgOxct1/Aacs tumours (Extended Data Fig. 8h). By contrast, 20:4(n-6) labelling was reduced only in sgOxct1/Aacs tumours (Extended Data Fig. 8i), and 22:4(n-6) labelling was elevated in sgBdh1 and sgOxct1 tumours but reduced in sgAacs and sgOxct1/Aacs tumours (Extended Data Fig. 8j). These results raise the possibility that in tumours in vivo, acetyl-CoA derived from the AACS route may be preferentially used for PUFA elongation, which may be consistent with a recent study suggesting that PUFA elongation by ketones requires AACS22.
Finally, we asked how β-OHB metabolism through these pathways might influence tumour growth. First, we found that loss of Bdh1, Oxct1, Aacs and Oxct1/Aacs had no effect on AL1376 PDAC subcutaneous tumour growth (Fig. 4i) but reduced the growth of orthotopic tumours in the pancreas (Fig. 4j). This may suggest that a factor in the pancreatic environment leads to a dependency on ketone metabolism for PDAC tumour growth. β-OHB concentrations averaged ~0.3 mM in tumour interstitial fluid (TIF) isolated from orthotopic tumours, which trended higher than levels found in subcutaneous TIF (Extended Data Fig. 9a), suggesting that β-OHB is available in the tumour microenvironment of orthotopic tumours. Moreover, quantification of total fatty acid levels showed that orthotopic TIF contained lower levels of some fatty acids, specifically monounsaturated fatty acids, compared to subcutaneous TIF (Extended Data Fig. 9b). Although these data do not establish whether impaired fatty acid synthesis from β-OHB was responsible for the slower growth of orthotopic AL1376 tumours lacking Bdh1, Oxct1, Aacs or Oxct1/Aacs, our results do suggest that ketone metabolism, including through the alternative AACS-dependent route, contributes to PDAC tumour growth in the pancreas.
In subcutaneously implanted B16 tumours, loss of ketone metabolism genes did not impair growth; in fact, loss of either Oxct1 or Aacs alone accelerated tumour growth, which may suggest that ketone metabolism may restrain tumour progression in some contexts (Fig. 4k,l). We asked whether there might be specific physiological contexts under which these ketone metabolism enzymes might be important for facilitating tumour growth. Previously, we demonstrated that caloric restriction (CR) decreases lipid levels and increases β-OHB levels in the tumour microenvironment2, and we reasoned that ketone metabolism may be important under these conditions. To test this hypothesis, mice bearing subcutaneous B16 control versus knockout tumours were fed a control diet or CR. As expected, CR reduced mouse body weights (Extended Data Fig. 9c,d) and decreased plasma glucose levels (Extended Data Fig. 9e). β-OHB concentrations in plasma collected from fasted mice under both diets averaged ~0.5 mM, reaching as high as ~3 mM in mice fed a CR diet (Extended Data Fig. 9f). CR also reduced the total plasma concentrations of many fatty acid species (Extended Data Fig. 9g). Finally, CR did not alter BDH1, OXCT1 or AACS protein levels in B16 tumours (Extended Data Fig. 9h).
A dependency on ketone metabolism for tumour growth was revealed under CR conditions. Although the growth of B16 sgNTC and sgBdh1 #1 tumours was minimally impaired by CR (Fig. 4m), the growth of B16 tumours lacking OXCT1 was significantly inhibited by CR (Fig. 4m). The growth of B16 sgAacs tumours in CR mice may trend towards a decrease (Fig. 4m) but was difficult to interpret because these tumours reached endpoint size in both diet groups by 7 days after diet administration, in contrast to 14 days for the sgNTC, sgBdh1 and sgOxct1 tumours. Finally, CR also inhibited the growth of B16 sgOxct1/Aacs tumours (Fig. 4n), albeit not to a greater extent than that observed for sgOxct1 tumours (Fig. 4m). We note that whether CR inhibited B16 tumour growth appears to correlate with whether loss of the ketone metabolism genes reduced β-OHB-derived cytosolic acetyl-CoA (Fig. 4g), in that Bdh1 loss did not reduce cytosolic acetyl-CoA labelling and did not sensitize tumours to CR, whereas Oxct1 or Oxct1/Aacs loss did lower cytosolic acetyl-CoA labelling and did sensitize tumours to CR. However, the physiological state under which our in vivo tracing experiments were performed was very different from that in CR mice, and our data do not establish whether impaired cytosolic acetyl-CoA synthesis from β-OHB specifically is responsible for improved responses to CR. We also note that the effect sizes observed with B16 knockout tumours were much less than those observed for AL1376 knockout tumours. Nevertheless, these results suggest that ketone metabolism, including through the alternative AACS-dependent route, may have a minor influence on B16 tumour growth in the context of a CR diet. Whether these pathways have a greater contribution to tumour growth in other cancer types or under other contexts will require further study.
In summary, in this study, we demonstrate that in cancer cells capable of metabolizing β-OHB, β-OHB can be a major source for the production of cytosolic acetyl-CoA, even when other key precursors such as glucose are available in excess. In addition to the canonical route of cytosolic acetyl-CoA synthesis through OXCT1-dependent production of citrate from the TCA cycle, we identify an alternative pathway in which β-OHB-derived acetoacetate in mitochondria can be exported to the cytosol and converted into cytosolic acetyl-CoA through AACS and cytosolic thiolases (Fig. 4o). This alternative routing allows β-OHB to bypass oxidation in the mitochondria to support its use as a major contributor to cytosolic acetyl-CoA. This feature distinguishes β-OHB from glucose and glutamine, as carbons from glucose and glutamine need to be routed through citrate to produce cytosolic acetyl-CoA. In this sense, β-OHB is similar to acetate, which is another alternative fuel for tumours that is directly converted into cytosolic acetyl-CoA by ACSS2 (refs. 23,24). These results have important implications for understanding how cancer cells maintain their cytosolic acetyl-CoA pool, particularly because ketones are typically absent from most standard culture media.
Notably, two recent studies22,25 have also described the contribution of ketones to fatty acid synthesis through AACS in the liver, highlighting how this alternative pathway functions in multiple tissues and cell types. A distinction between ketone metabolism in liver tissue versus cancer cells is that the liver, as a ketogenic tissue, does not express OXCT1 to prevent ketolysis and ketone oxidation. By contrast, cancer cells that can metabolize ketones can do so through both the OXCT1-dependent and the AACS-dependent routes, and this supports the use of β-OHB as a major contributor to cytosolic acetyl-CoA, even when glucose is present. Of note, our data suggest that the relative contribution of the OXCT1-dependent route versus the AACS-dependent route may be cell-line-dependent. In AL1376 cells, the AACS route appears to be more dominant, given that the shift to the left in the palmitate MID and loss in cytosolic acetyl-CoA labelling was greater in the Aacs knockout cells than in the Oxct1 knockout cells (compare Fig. 3k,l with Fig. 3g,h). Conversely, the OXCT1 route appears to be more dominant in B16 cells (compare Extended Data Fig. 3g,h with Extended Data Fig. 3k,l). The factors that determine which pathway is more dominant and whether the relative activities of these two routes might be regulated in certain contexts remain to be determined.
Finally, cytosolic acetyl-CoA has multiple downstream fates, including fatty acid synthesis26, cholesterol synthesis27 and protein and histone acetylation21,28. How the different routes of cytosolic acetyl-CoA production from β-OHB affect these downstream fates remains an open question, as does whether any of these downstream fates are particularly important for cancer progression under specific contexts. Our identification of an alternative pathway for β-OHB-derived cytosolic acetyl-CoA production will aid future studies that seek to better define how ketone body metabolism influences diverse downstream cellular processes to alter cancer progression.
Source link