Glucagon-like peptide-1 (GLP1) and glucose-dependent insulinotropic polypeptide (GIP) potently amplify insulin secretion and influence satiety to reduce food intake1,2,3. Stabilized GLP1R agonists were approved for the treatment of type 2 diabetes in 2008 (ref. 4) and at higher doses for overweight and obesity in 2017 and 2018 (ref. 5). Less attention has been paid to the GIP axis, as multiple studies showed that either genetic ablation or pharmacological blockade of GIPR protects against body weight gain6, and that GIPR agonism has minimal effects on glycemia in patients with type 2 diabetes7, potentially owing to GIPR downregulation8. However, dual agonists such as tirzepatide combine elements of GLP1R and GIPR agonism, such that the full benefits of both hormone axes are realized9.
Although the pharmacological mechanisms underlying dual GLP1R/GIPR agonist efficacy are well established10,11,12, less is known about the cellular substrates that underlie actions on pancreatic hormone secretion as well as food intake. GLP1R and GIPR are low-abundance cell surface proteins, and their instability in solution makes it difficult to raise antibodies against specific epitopes, rendering their immunohistochemical visualization challenging13. Specific antibodies exist for GLP1R, but no reliable commercial antibody exists yet for the detection of GIPR in primary cells and tissues13. Compared to antibodies, GLP1R-Cre and GIPR-Cre reporter mice are sensitive, but only identify cells that express(ed) GLP1R/GIPR transcripts14,15. Finally, animals harbouring a self-labelling enzyme on the GLP1R amino terminus allow facile visualization using a range of fluorophores, although this model is restricted to rodents16. No approach to date provides information on GLP1Rs and GIPRs specifically accessed or bound by a dual agonist.
To circumvent these issues, we previously developed LUXendins and sGIPs17,18,19, which are specific probes against GLP1R and GIPR, respectively. Using these probes, we confirmed that GLP1R is largely a β cell-specific protein marker18,20, whereas GIPR is expressed in both β cells and α cells within the islet17. However, we were unable to make any inferences about dual agonist binding sites within the islet and brain, as GLP1R and GIPR probes might target different receptor (sub)populations. Dual agonism might also influence GIPR/GLP1R localization, expression and access through effects in addition to those mediated by GLP1R and/or GIPR (ant)agonism alone; for example, release of paracrine mediators and functional selectivity21,22,23. Although fluorescent tirzepatide has been reported12, the probe was not pharmacologically validated for its specificity at GLP1R/GIPR.
Design of daLUXendin544 and daLUXendin660
Structure–activity relationships and cryo-electron microscopy show that the binding affinity and efficacy of GLP1R agonists, GIPR agonists and dual agonists are determined by amino acids closer to the N-terminus24,25,26,27. We thus reasoned that carboxy-terminal functionalization with a fluorophore would be well tolerated, especially because this end is not commonly resolved in structural interpretations. Given that tirzepatide possesses a C-terminal serine, we decided to generate an S39C mutant, allowing the late-stage addition of a fluorophore (Fig. 1a,b). daLUXendin544 and daLUXendin660 were obtained using cysteine-maleimide ‘click’ chemistry to install Cy3 and Cy5 fluorophores, respectively, in yields of ~50%. iso-butyric amino acid was incorporated into the structure instead of alanine at position two to confer degradation resistance against DPP-IV28 (Fig. 1a,b). Spectral properties, compound purity and compound characterization are shown in Supplementary Fig. 1 and Supplementary Note 1.
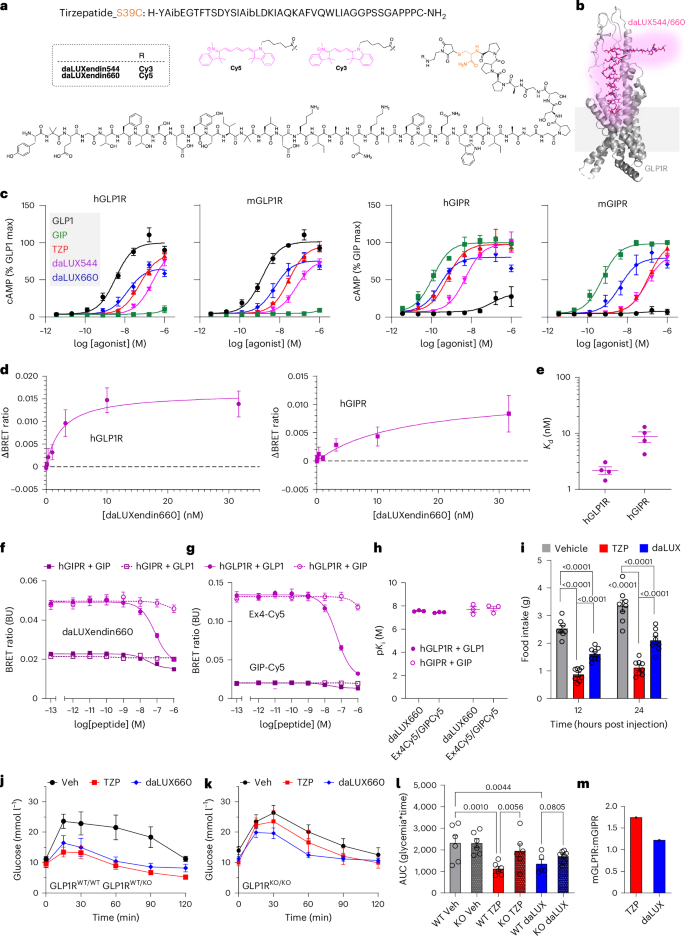
a,b, Tirzepatide, modified at the C-terminus with S39C to allow maleimide conjugation with either Cy3 or Cy5 (model obtained from cryo-electron microscopic structure of non-acyl tirzepatide bound to activated GLP1R in complex with Gs) (PDB 7VBI)26. c, cAMP responses to GLP1, GIP, tirzepatide (TZP), daLUXendin544 (daLUX544) and daLUXendin660 (daLUX660) in AD293 cells expressing either hGLP1R, mGLP1R, hGIPR or mGIPR (n = 4 independent repeats). d,e, Binding affinity of daLUXendin660 against hGLP1R and hGIPR (d), with Kd values (e) in a separate bar graph (one-site fit specific binding) (n = 3 independent repeats). f,g, Competition binding for daLUXendin660 (f) and either Ex4-Cy5 or GIP-Cy5 (g) at hGLP1R and hGIPR versus GLP1 and GIP (one-site fit Ki) (n = 3 independent repeats). h, pKi values for daLUXendin660, Ex4-Cy5 and GIP-Cy5 at hGLP1R and hGIPR versus GLP1 and GIP (n = 3 independent repeats). i, Mice were fasted 3 h before the onset of the dark phase. At dark onset, mice were injected subcutaneously with either vehicle, daLUXendin660 (daLUX; 10 nmol kg−1) or tirzepatide (10 nmol kg−1), and then food was returned. Graph showing cumulative food intake 12 h and 24 h post injection (n = 8 mice per investigated state) (repeated-measures two-way ANOVA with Šídák’s multiple comparisons test). j, Tirzepatide and daLUXendin660 similarly lower glucose in GLP1RWT/WT and GLP1RWT/KO mice during glucose tolerance testing (n = at least five mice per investigated state). k,l, Efficacy of tirzepatide and daLUXendin660 is reduced in GLP1RKO/KO mice (k), shown also by area under the curve (AUC) (l) (n = at least five mice per investigated state) (one-way ANOVA with two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli). m, mGLP1R:mGIPR selectivity is lower for daLUXendin660 versus tirzepatide (selectivity is calculated using the mean AUC ratio from at least five GLP1RWT/WT and five GLP1RKO/KO mice). Bar and line graphs show means; error bars, s.e.m. ***P < 0.001. Exact P values are displayed on each graph.
Pharmacology of daLUXendin544 and daLUXendin660
AD293 cells transfected with either human GLP1R (hGLP1R), mouse GLP1R (mGLP1R), human GIPR (hGIPR) or mouse GIPR (mGIPR) were used to determine cAMP signalling potency of daLUXendin544 and daLUXendin660 versus unmodified (acylated) tirzepatide, GIP and GLP1. At both hGLP1R and mGLP1R, tirzepatide and its fluorescent analogues showed reduced potency compared to GLP1, with the potency rank order favouring daLUXendin660 > tirzepatide > daLUXendin544 (Fig. 1c) (Extended Data Table 1). As previously reported for tirzepatide11, there were species differences for GIPR signalling with each of the dual agonist ligands tested, with all showing a larger loss of potency at the mGIPR than at the hGIPR relative to native hGIP (Fig. 1c) (Extended Data Table 1 and Supplementary Table 1). Of note, even though daLUXendin544 showed globally reduced potency compared to daLUXendin660, half-maximum effective concentration (EC50) ratios suggested that both ligands show similar GIPR:GLP1R selectivity of ~2:1 (that is, favouring GIPR) for human receptors and ~1:2 (that is, favouring GLP1R) for mouse receptors. By comparison, tirzepatide showed GIPR:GLP1R selectivity of ~3:1 for human receptors and ~1:8 for mouse receptors in our assays.
daLUXendin660 showed nM binding affinity at both hGLP1R and hGIPR, although assessment of the daLUXendin544 dissociation constant (Kd) was precluded by interference with the BRET signal (Fig. 1d,e) (Supplementary Table 2). Competition assays showed that binding affinity (pKi) for hGLP1/hGLP1R and hGIP/hGIPR was similar when using daLUXendin660 as a probe compared to Ex4-Cy5 and GIP-Cy5, further confirming strong receptor binding (Fig. 1f–h) (Supplementary Table 3).
Therefore, based upon cAMP assays and binding affinity, daLUXendin544 and daLUXendin660 are potent GLP1R/GIPR dual agonists that advantageously show approximately fourfold less selectivity for mouse GLP1R over GIPR compared to tirzepatide.
daLUXendin660 shows less selectivity for GLP1R:GIPR in vivo
To establish whether in vitro pharmacology is mirrored by in vivo efficacy, mice were dosed with either tirzepatide or daLUXendin660 at 10 nmol kg−1 to maximally engage mGLP1R and mGIPR11,29. At this dose, daLUXendin660 and tirzepatide both reduced food intake during the dark phase and over a cumulative 24 h period. At 12 h and 24 h post injection, tirzepatide demonstrated ~40% greater efficacy in driving anorexia, presumably because of its acylation and plasma binding (Fig. 1i). Likewise, both tirzepatide and daLUXendin660 exerted substantial glucose-lowering effects in Glp1rWT/WT and Glp1rWT/KO mice, with similar area under the curves (Fig. 1j–l). Both ligands partially maintained their efficacy in Glp1rKO/KO mice, reflecting continued engagement of mGIPR at doses >1–3 nmol kg−1 (Fig. 1j–l). However, Glp1r knockout was less effective in daLUXendin660-treated versus tirzepatide-treated mice, demonstrating increased mGIPR dependence for daLUXendin660, as expected from in vitro functional selectivity data (Fig. 1j–m). Results should, however, be interpreted in light of increased mGIPR sensitivity or expression in Glp1rKO/KO mice11. Together, these data confirm that daLUXendin660 and tirzepatide both lower blood glucose and reduce food intake.
Cell labelling with daLUXendin544 and daLUXendin660
In line with the cAMP results, daLUXendin544 led to concentration-dependent labelling of SNAP-hGIPR:AD293 cells, overlapping with cell-impermeable SBG-Oregon Green (OG) SNAP label (Extended Data Fig. 1a,b)30. The brightest labelling was detected at 50 nM–5 µM, corresponding to the cAMP max (Extended Data Fig. 1a,b). The strongest and most specific membrane labelling, without evidence of altered cell morphology (Extended Data Fig. 1a–c), was observed with 500 nM daLUXendin544; hence, this concentration was selected for experiments in cell lines and tissue. No labelling was observed in non-transfected AD293 cells (Extended Data Fig. 1d). Both daLUXendin544 and daLUXendin660 showed colocalization with cells transfected with SNAP_GIPR and Halo_GLP1R, determined using orthogonal cell-impermeable SNAP (SBG-OG) and Halo (CA-Sulfo549 or CA-Sulfo646) labels31 (Extended Data Fig. 1e,f). Therefore, daLUXendin544 and daLUXendin660 are highly specific dual agonist probes suitable for cell labelling.
daLUXendins label endogenous GIPR and GLP1R
MIN6-CB4 β cells were labelled with both daLUXendin660 and the specific GLP1R probe LUXendin551 (refs. 18,19). Demonstrating specificity for GLP1R, LUXendin551 signal colocalized with daLUXendin660 (Extended Data Fig. 2a), probably owing to labelling of different GLP1R pools (see below). However, not all daLUXendin660 signal colocalized with LUXendin551, presumably owing to GIPR labelling, as well as increased GLP1R internalization (Extended Data Fig. 2a). To investigate this possibility further, MIN6-CB4 β cells were incubated with daLUXendin660 and the specific GIPR probe sGIP549 (ref. 17) (Extended Data Fig. 2b). Demonstrating specificity for GIPR, sGIP549 signal colocalized with daLUXendin660, whereas not all daLUXendin660 signal colocalized with sGIP549, corresponding to GLP1R labelling (Extended Data Fig. 2b). Therefore, daLUXendin660 labels both endogenous GLP1R and GIPR in MIN6-CB4 β cells.
We next repeated studies in isolated mouse islets. To circumvent competition between the various probes at GLP1R/GIPR, we used GLP1RSNAP/SNAP mice in which a SNAP-tag enzyme self-label is knocked into the extracellular domain of the endogenous GLP1R16, allowing GLP1R to be visualized without influencing orthosteric binding. Using this model, colocalization could be detected between the cell-impermeable SNAP label BG-Sulfo646 and daLUXendin544 (Extended Data Fig. 2c). We noticed that daLUXendin544 retained significant levels of GLP1R at the cell surface, as shown by the full width at half maximum (Extended Data Fig. 2d,e). By contrast, daLUXendin544 did not exhibit any detectable membrane labelling in α cells, presumably because they express little to no GLP1R transcript or protein (also used to identify α cells versus β cells) (Extended Data Fig. 2f,h)15,18,20, so α cell labelling is largely by GIPR. Using the GIPR probe sGIP648, daLUXendin544 was found to strongly label GIPR in mouse islets (Extended Data Fig. 2g,h), also apparent from studies with the GLP1R probes and GLP1R SNAP labels. Lastly, daLUXendin544 labelling was reduced ~50% in GLP1RKO/KO islets, with a further reduction in labelling seen in the presence of a saturating concentration of GIPAib2 (1 µM) (Extended Data Fig. 2i,j).
daLUXendins label most endocrine cell types within the islet
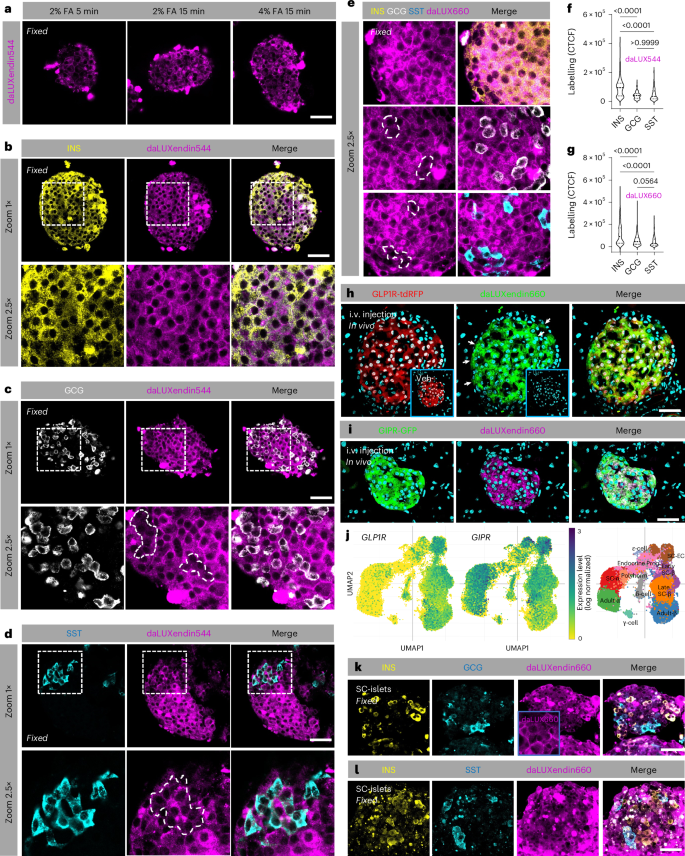
To allow post hoc protein labelling and identification of cell types in complex tissue, we optimized a fixation protocol that retained strong and specific daLUXendin544 and daLUXendin660 labelling post-fixation with either 2% or 4% formalin for 15 min (Fig. 2a). Islets were labelled with daLUXendin544 before formalin-fixation, and immunostaining with antibodies against either insulin, glucagon or somatostatin to identify β cells, α cells or δ cells, respectively. As expected, given their abundant GLP1R and GIPR expression, β cells were strongly labelled with daLUXendin544 (Fig. 2b). Confirming results in live islets, α cells showed weaker but detectable daLUXendin544 labelling restricted to the cytoplasm (Fig. 2c), probably reflecting their abundant GIPR expression and largely absent GLP1R protein expression17,18,20. δ cells also showed weaker labelling versus β cells (Fig. 2d). However, labelling was observed both in the cytoplasm and membrane, which might reflect low-moderate expression of GIPR and GLP1R protein (Fig. 2d). Identical results were obtained with daLUXendin660 (Fig. 2e). Quantification of daLUXendin544/660 labelling is shown in Fig. 2f,g. Demonstrating efficacy of daLUXendin660 in vivo, islets in GLP1R-tdRFP and GIPR-GFP reporter mice were strongly and specifically labelled 60 min following intravenous injection with 100 nmol kg−1 probe but not vehicle (Fig. 2h,i). As for isolated islets, both GLP1R+ and GLP1R− cells were labelled in vivo, the latter probably representing GIPR+ cells (that is, α cells) (Fig. 2h).
a, Following labelling of live islets, daLUXendin660 can be fixed using either 2% or 4% formalin (FA) for 15 min (n = at least five islets from three mice). b, daLUXendin544 labels insulin-positive (INS+) cells throughout the mouse islet (n = 44 islets from five mice). c,d, daLUXendin544 labels glucagon-positive (GCG+) and somatostatin-positive (SST+) cells throughout the islet, although to a lesser extent than INS+ cells (INS, 44 islets from five mice; GCG, 25 islets from seven mice; SST, 29 islets from seven mice). e, daLUXendin660 displays a similar labelling pattern to daLUXendin544 in mouse islets (INS, 43 islets from five mice; GCG, 30 islets from seven mice; SST, 27 islets from seven mice). f,g, Quantification of daLUXendin544 (f) and daLUXendin660 (g) labelling intensity in INS+, GCG+ and SST+ cell populations (Kruskal–Wallis test with Dunn’s multiple comparisons test). CTCF, corrected total cell fluorescence. h, Intravenous (i.v.) injection of daLUXendin660, but not vehicle (inset), labels GLP1R+ cells in islets from GLP1R-tdRFP reporter mice (arrows show GLP1R− but daLUXendin660+ cells) (n = at least nine islets). i, As in h, but with GIPR-GFP reporter mice (n = 9 islets). j, Uniform manifold approximation and projection (UMAP) plots showing GLP1R and GIPR expression in early and late SC-islet endocrine cell populations (taken from a previous publication35, and plotted using Single Cell Portal54, study SCP1526). k,l, daLUXendin660 labels INS+, GCG+ and SST+ cell populations in SC-islets (inset in k, a separate non-immunostained SC-islet showing membrane and cytoplasmic daLUXendin660 signal). Scale bar, 53 µm. Violin plots show min–max and median. Exact P values are displayed on each graph.
daLUXendins label most cell types in human stem cell islets
Fluorescent labelling of low-abundance proteins in human islets is limited by high levels of autofluorescence, in particular from lipofuscin accumulation32. In addition, human islet preparations display heterogeneity in response to GLP1R/GIPR agonist, probably reflecting variable GLP1R/GIPR expression as well as time in culture. To circumvent these issues, we instead turned to human induced pluripotent stem (iPS) cell-derived islet-like structures (SC-islets), which are therapeutically important as a cell therapy for type 1 diabetes33,34. Analysis of published single-cell RNA sequencing data35 revealed GIPR and GLP1R in early and late β-like cells as well as δ cells, and GIPR in early and late α cells (Fig. 2j). daLUXendin660 labelling was qualitatively similar to that observed in mouse islets, with cells displaying two similar labelling patterns: membrane labelling + cytoplasmic labelling or cytoplasmic staining alone (Fig. 2k, inset). In keeping with the transcriptomic data, immunostaining revealed daLUXendin660 labelling in insulin (INS+), glucagon (GCG+) and somatostatin (SST+) cell populations (Fig. 2j–l). Some daLUXendin660 labelling was detected in INS−/GCG−/SST− cells, which were presumably GLP1R/GIPR-expressing enteroendocrine cells that are present in most differentiations (Fig. 2j–l). Similar to mouse islets, the strongest daLUXendin660 labelling was seen in β cells, with weaker but detectable labelling in α cells and δ cells (Fig. 2k,l) (corrected total cell fluorescence, 3.60 × 105 ± 2.58 × 105 versus 2.30 × 105 ± 1.59 × 105 versus 2.16 × 105 ± 1.46 × 105 AU, β cells versus α cells versus δ cells, respectively; P < 0.0005 one-way ANOVA and Šídák’s multiple comparisons test) (n = 12 SC-islets from two differentiations).
daLUXendin660+ optimized for long-term dosing
To expand the utility of daLUXendin660 for long-term dosing, we installed a C20 di-acid lipid chain on lysine at position 20 to confer albumin binding. The new molecule, termed daLUXendin660+, performed similarly to daLUXendin660 in pharmacology and receptor binding assays, demonstrating that acylation and lipid modification is well tolerated (Extended Data Fig. 3a–d) (Extended Data Table 2 and Supplementary Table 4). No differences in pancreatic islet cell labelling could be detected between daLUXendin660 and daLUXendin660+ applied to the same islet batch (that is, INS > GCG and SST) (Extended Data Fig. 3e–h).
daLUXendins label GLP1R+ and GIPR+ neurons
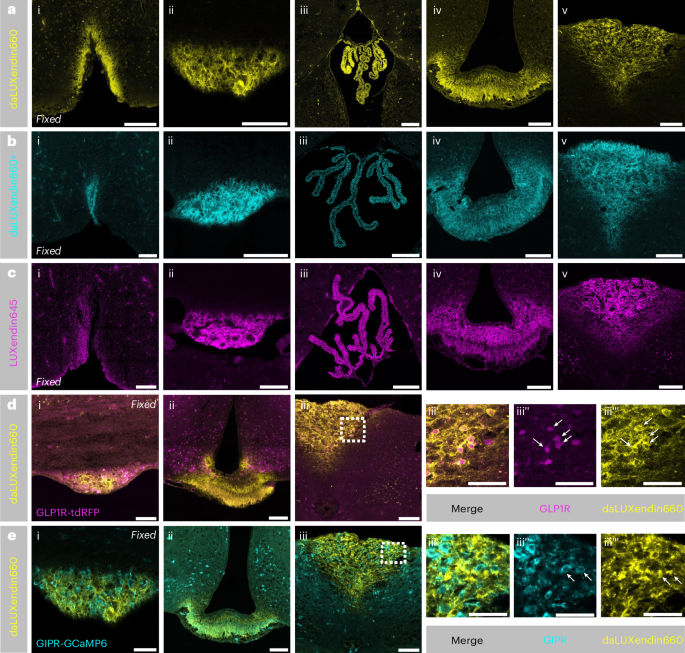
Following intravenous injection (100 nmol kg−1), daLUXendin660 signal could be readily detected in the circumventricular organs and choroid plexus but not in other areas of the brain (Fig. 3a). The circumventricular organs are characterized by an incomplete blood–brain barrier and include areas such as the median eminence, area postrema, subfornical organ and organum vasculosum of the lamina terminalis, brain regions known to bind GLP1R and GIPR (ant)agonists17,18. We did not observe any difference in the extent of brain penetration between daLUXendin660, daLUXendin660+ and the single GLP1R agonist LUXendin645 (Fig. 3a–c). To establish which incretin receptor-expressing neurons are labelled with daLUXendin660, we performed intravenous injection (100 nmol kg−1) of daLUXendin660 in transgenic mice Cre-dependently expressing fluorescent reporters in either GLP1R-expressing cells (GLP1R-tdRFP) or GIPR-expressing cells (GIPR-GCaMP6). Clear colocalization of daLUXendin660 with GLP1R (Fig. 3d) and GIPR (Fig. 3e) neurons was observed in the area postrema.
a–c, Intravenous administration of 100 nmol kg−1 daLUXendin660 (n = 3 mice) (a), 100 nmol kg−1 daLUXendin660+ (n = 2 mice) (b) and subcutaneous administration of 100 nmol kg−1 LUXendin645 (GLP1R antagonist) (n = 5 mice) (c) led to staining in circumventricular organs of the brain and the choroid plexus. Shown are the organum vasculosum of the lamina terminalis (i), subfornical organ (ii), choroid plexus (iii), median eminence (iv) and area postrema (v). d,e, Intravenous administration of 100 nmol kg−1 daLUXendin660 in a GLP1R-tdRFP mouse (n = 1) (d) or GIPR-GCaMP6 mouse (n = 2) (e) labels GLP1R-expressing and GIPR-expressing cells, respectively (indicated by white arrows). Shown are the subfornical organ (i), median eminence (ii), area postrema (iii) and zoom-in of area postrema with merged (iii’), GLP1R-tdRFP/GIPR-GCaMP6 (iii’’) and daLUXendin660 (iii’’’) signals. Note that images in e are from the same mouse as in a but with GIPR-GCaMP6 signal overlaid. Scale bars, 100 µm.
The strength and extent of labelling does not necessarily equate to agonist efficacy, especially for peptidic ligands that diffuse freely through fenestrated vessels of the median eminence into the ventricular system22,36. To assess the accessibility of daLUXendin660 binding sites from the cerebrospinal fluid, we performed intracerebroventricular injection of the probe. Intracerebroventricular administration of daLUXendin660 demonstrated clear penetration from the cerebrospinal fluid into the brain parenchyma. Strong labelling was observed in tanycytes—identified through vimentin immunostaining—within the third ventricle (Extended Data Fig. 4a). daLUXendin660 labelling in tanycytes was localized to the apical surface as well as cellular processes extending into the brain parenchyma (Extended Data Fig. 4b). Neurons identified as GLP1R+ in GLP1R-tdRFP reporter mice were co-labelled with daLUXendin660 in the vicinity of the ventricles (Extended Data Fig. 4c). Modest colocalization in GIPR+ neurons was evident in GIPR-GCaMP6 reporter mice (Extended Data Fig. 4d). A small number of neurons positive for both GIPR and GLP1R were labelled with daLUXendin660 (Extended Data Fig. 4e).
daLUXendin660 engages highly ordered GLP1R/GIPR nanodomains
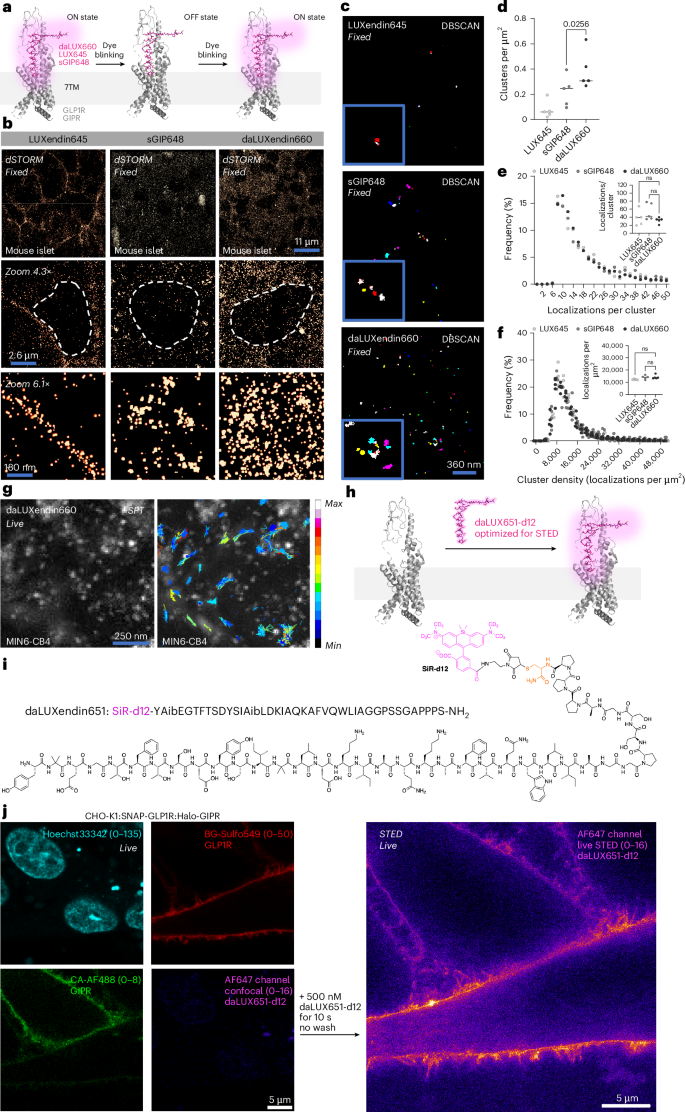
GPCRs possess higher organization at the cell membrane and within the cell, forming nanodomains that are important for signalling16,37,38. We therefore set out to determine how endogenous GLP1R/GIPR nanodomains might be targeted by single agonists versus a dual agonist in primary tissue; that is, pancreatic islets. We first established nanodomain organization in the non-stimulated but bound state using LUXendin645, a GLP1R antagonist coupled to Cy5. Following 60 min incubation with LUXendin645, fixation and dSTORM nanoscopy of whole islets, we confirmed our previous data showing that non-stimulated GLP1R form discrete nanodomains16,18 (Fig. 4a,b). The GIPR agonist sGIP648 also labelled discrete receptor nanodomains (GIPR), as defined by clustering (Fig. 4b–d). daLUXendin660, however, engaged more ordered receptor nanodomains compared to the GIPR agonist alone, with high levels of clustering probably reflecting increased receptor interactions (Fig. 4b–d). Localization number per cluster, as well as the density of each cluster, were similar across all ligands examined (Fig. 4e,f). Together, these data suggest that tirzepatide either engages GLP1R into clustering or increases interactions between GLP1R and GIPR, thus contributing to its unique nanodomain arrangement. Owing to the long acquisition times needed for localization counting, dSTORM is best suited to fixed tissue. We therefore set out to show the applicability of daLUXendin660 for TIRF-based GLP1R/GIPR single particle tracking at the cell membrane in live MIN6-CB4 cells (Fig. 4g). To open up super-resolution live imaging approaches, such as STED, we replaced the C-terminal Cy5 with fluorogenic deuterated silicon rhodamine (SiR-d12)39 to create daLUXendin651-d12 (Fig. 4h,i and Supplementary Fig. 1). Labelling with daLUXendin651-d12 was too dim to visualize endogenous receptor but was sufficient to map GLP1R in fixed CHO-K1:SNAP-GLP1R:Halo-GIPR cells. daLUXendin651-d12 was able to image GLP1R below the diffraction limit and could be recorded over multiple frames (Supplementary Fig. 2). Following direct application of daLUXendin651-d12 to CHO-K1:SNAP-GLP1R:Halo-GIPR cells, far-red cell surface signal from SiR-d12 became rapidly visible (10 s) in live STED on the cell surface, colocalizing with BG-Sulfo549 and CA-AF488 (Fig. 4j).
a, Schematic showing single-molecule labelling and localization strategy for GLP1R (LUXendin645; LUX645), GIPR (sGIP648) and GLP1R/GIPR (daLUXendin660) (PDB 7VBI)26. b, Representative dSTORM images show different single-molecule densities and organization (30 nm) of LUX645-bound GLP1R, sGIP648-bound GIPR and daLUXendin660-bound GLP1R/GIPR across an islet cell population (nucleus is bounded by dashed line) (n = 5 islets from three mice). c,d, daLUXendin660 labels more GLP1R/GIPR clusters compared to either LUX645 or sGIP648, shown by representative images (c) and bar graph (d) (n = 5 islets from three mice) (one-way ANOVA with two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli). e, Distribution and mean (inset) localization per cluster are similar across LUX645, sGIP648 and daLUXendin660 probes (n = 5 islets from three mice) (one-way ANOVA with Šídák’s multiple comparisons test). f, As in e, but for cluster density (one-way ANOVA with Šídák’s multiple comparisons test). g, Single particle tracking of daLUXendin660-labelled GLP1R/GIPR, showing min–max displacement (n = 5 cells, two independent repeats). h,i, SiR-d12 can be installed on daLUXendin (h) to create daLUXendin651-d12 (i), optimized for STED nanoscopy. j, Confocal snapshots showing CHO-K1:SNAP-GLP1R:Halo-GIPR cells labelled for GLP1R (BG-Sulfo549) and GIPR (CA-AF488) before live STED imaging of daLUXendin651-d12 labelling (n = 5 wells). Scale bars are provided on each figure. ns, non-significant. Exact P values are displayed on each graph.
In the present study, we synthesize and validate daLUXendins, red and far-red GLP1R/GIPR dual agonist probes. Nanomolar concentrations of daLUXendin specifically label GLP1R/GIPR in live cells, allowing endogenous binding sites and cellular targets for dual agonists such as tirzepatide to be visualized and interrogated. daLUXendin labelling can be achieved in most cells and tissues with a simple 1 h incubation or injection, followed by washing and analysis. Advantageously, daLUXendins show minimal loss in signal intensity when formaldehyde-fixed, allowing co-labelling for other cellular or neuronal proteins and markers.
A key aspect of tirzepatide efficacy is increased potency and selectivity at human GIPR compared to GLP1R, with approximately 70% of activity at GIPR10,11,12. In mice, however, tirzepatide is selective for GLP1R over GIPR, and its insulinotropic and overall therapeutic efficacy is equivalent or non-superior to semaglutide (GLP1R agonist)10,11. Although this is unlikely to have any bearing on GLP1R and GIPR accessed and bound by tirzepatide, it remains a limitation to the use of preclinical mouse models to understand GLP1R/GIPR dual agonism. Notably, daLUXendin544 and daLUXendin660 displayed less selectivity for GLP1R over GIPR in mouse (1:2 GIPR:GLP1R) compared to tirzepatide (1:8 GIPR:GLP1R). Although the exact mechanisms underlying the differences between daLUXendin544/660 and tirzepatide remain unknown, our previous studies showed that fluorophore modification can increase GLP1R agonist efficacy18. Therefore, daLUXendin660 could be a useful tool for probing dual agonist biology in the mouse.
Within the pancreatic islet, tirzepatide labelled β cells > α cells = δ cells, in keeping with known protein or transcript expression levels for GLP1R and GIPR (as previously reviewed21). Although studies have shown that tirzepatide stimulates secretion of insulin, somatostatin and glucagon11,21, the cellular substrates have so far remained elusive. The observation that tirzepatide labels β cells, δ cells and α cells suggests both direct and paracrine modes of action. Given that α cells express GIPR >> GLP1R, direct effects of tirzepatide on glucagon secretion are probably mediated by GIPR signalling, a known stimulator of α cell function40,41. By contrast, δ cells express GIPR > GLP1R, inferring a predominant role for GIPR in tirzepatide-stimulated somatostatin secretion, with some GLP1R contribution. β cells express GLP1R = GIPR, but increased affinity of tirzepatide for human GIPR10 is likely to disproportionately contribute to insulin secretion. As well as exerting direct effects on islet cell function, tirzepatide is also likely to stimulate α cell-to-β cell and δ cell-to-β cell communication, further regulating insulin release (as previously reviewed21,42). At the same time, tirzepatide might engage β cell-to-α cell or δ cell-to-α cell communication to provide a brake on glucagon secretion.
Peripherally injected daLUXendin660 was able to label the pancreas as well as readily access the median eminence, organum vasculosum of the lamina terminalis, subfornical organ and area postrema. The extent of daLUXendin660 labelling was similar to that seen for a GLP1R antagonist, LUXendin645, as well as a GIPR agonist, sGIP648 (refs. 17,18). We therefore speculate that the superior efficacy of GLP1R/GIPR dual agonism versus single GLP1R agonism reflects the specific neuronal and supporting cell populations that are activated rather than the extent of brain access. When administered directly into the ventricular space of the brain, daLUXendin660 labelled GLP1R+ and GIPR+ neurons in the vicinity of the ventricles, as well as periventricular cells such as third ventricular tanycytes. Periventricular cell labelling is a common feature across GLP1R/GIPR (dual) (ant)agonists, pointing to a critical role for this cell type in the regulation of food intake and possibly the ligands themselves43.
Outside of conventional pharmacology, it remains unknown how daLUXendin660 interacts with GLP1R/GIPR in cells that endogenously express both receptors. Single-molecule localization microscopy showed that daLUXendin660 densely labelled GLP1R/GIPR clusters or nanodomains in islets, which was in addition to that seen with a GIPR agonist alone. Therefore, daLUXendin660 probably influences either GLP1R clustering or GLP1R and GIPR localization to increase nanodomain formation. We speculate that GIPR and GLP1R signalling within or between cell nanodomains, rather than simple signal summation across the cytoplasm, contributes to tirzepatide efficacy, as recently shown for GLP1 and β2-adrenergic receptors44.
Tirzepatide is just one of many emerging dual and triple agonists that might exhibit different receptor engagement profiles. Given that we are still piecing together single agonist function45, daLUXendin provides a powerful tool to understand dual agonist receptor engagement, brain or organ access and receptor synergism. Owing to the structural similarities between dual and triple agonists, in the future, daLUXendins can be easily modified to incorporate a glucagon receptor agonist component.
The study has a number of limitations. Firstly, tirzepatide and daLUXendins are different molecules, and detailed pharmacokinetic profiles were not determined. Therefore, we cannot categorically state that brain areas or cell types accessed by tirzepatide in humans and rodents would be similar to those accessed by daLUXendins in rodents. Secondly, we did not provide any data on human islets. We have noticed heterogeneity in GLP1R/GIPR expression, probably reflecting culture time and donor variability, compounded by high levels of lipofuscin autofluorescence32. Thirdly, we restricted daLUXendin544/660/651-d12 to Cy3, Cy5 and SiR-d12 fluorophores, as they are well suited for complex tissue labelling16,18,19. In the future, the daLUXendin colour palette could be extended to include blue-near-infrared and even epitope tags to allow signal amplification.
In summary, daLUXendin544/660 are highly specific probes that highlight dual agonist target cells within complex tissues such as the pancreatic islet and brain. We envisage that daLUXendin544/660 will provide interrogable mechanisms underlying dual agonist efficacy.
Source link