A tidy textbook definition of life has never existed, yet most biology lessons still lean on the idea that living things must grow, make energy, and reproduce on their own.
That simple checklist leaves viruses outside the club, since their genetic shells activate only inside another organism and go dormant again when drifting alone.
Ryo Harada of Dalhousie University and colleagues discovered a creature that forces us to redraw those lines on the fly.
Their oddball microbe, provisionally named Sukunaarchaeum mirabile, was hiding in DNA scraped from a single plankton species off the Japanese coast.
Sukunaarchaeum virus line blurs
Biologists once leaned on André Lwoff’s dictum that “an organism is constituted of cells.” That rule pushed viruses into a separate realm of particles.
That neat boundary already looked wobbly when giant viruses surfaced in the early 2000s, flaunting genomes larger than some bacteria.
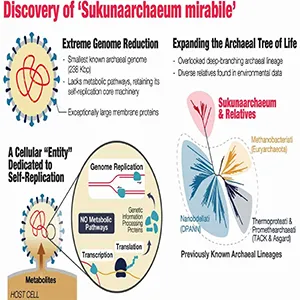
Sukunaarchaeum muddies the waters further. It is undeniably cellular, yet its playbook borrows many viral tricks. It retains the genes for building its own ribosomes and messenger RNA – parts no virus carries – but appears to outsource nearly every other task to a host cell.
A record-breaking small genome
The new organism’s entire genome fits into 238,000 base pairs – roughly the length of a medium-sized magazine article.
For comparison, the minimalist archaeon Nanoarchaeum equitans holds the previous cellular record at about 490,000 base pairs, already microscopic by prokaryotic standards.
Viruses can be both larger and smaller, yet they never lug around the full toolkit for protein synthesis. That is why Harada’s team calls Sukunaarchaeum “a cellular entity retaining only its replicative core.”
A virus-like microbe emerges
“Its genome is profoundly stripped-down, lacking virtually all recognizable metabolic pathways, and primarily encoding the machinery for its replicative core: DNA replication, transcription, and translation,” wrote Harada and co-authors in their report.
The code resembles a viral instruction manual more than a self-sufficient microbe.
Yet the creature sits within Archaea, one of the three great domains of life – not among viruses. Phylogenetic trees place it on a deep branch so distant from known groups that the authors propose creating a new phylum.
Sukunaarchaeum and borrowed genes
The team stumbled on the microbe while sequencing DNA from the dinoflagellate Citharistes regius. Nested in that plankton’s genetic debris was a tight loop of foreign DNA that refused to match any catalogued species.
Marine symbiosis can be intimate: some plankton rely on bacterial partners for vitamins, while others house entire algal cells that photosynthesize for them.
Sukunaarchaeum appears to take this intimacy to the extreme, shaving off every gene it can afford to lose and leaning on its host for nearly everything else a cell needs to stay alive.
Redrawing the tree of life
Because its ribosomal genes remain intact, the organism qualifies as cellular under the classic molecular litmus test.
Yet its pared-down metabolism likely prevents it from harvesting nutrients, producing ATP, or fixing carbon without help – behaviors normally reserved for viral passengers.
Scientists debate whether life is a binary label or a spectrum; Sukunaarchaeum pushes the spectrum view to the forefront.
The find suggests that many other stealth lineages may be hiding in environmental sequencing data, dismissed as contaminants or viral oddities.
Defining the meaning of “alive”
Words such as “alive” steer funding, public health policy, and even planetary protection rules for space probes.
If more organisms like Sukunaarchaeum exist, biosecurity protocols that screen only for free-living microbes could miss entire classes of symbiotic parasites.
The discovery also sharpens a practical question: What genetic load is the bare minimum for a cell to function? Synthetic biology groups pursuing engineered minimal cells may mine this archaeon’s blueprint for clues.
“The discovery of Sukunaarchaeum pushes the conventional boundaries of cellular life and highlights the vast unexplored biological novelty within microbial interactions,” wrote the researchers.
Harada’s team suspects that extreme genome pruning evolved because the host environment guaranteed nutrients, letting redundant pathways decay.
Paleobiologists see a glimpse of early evolution, when ancient cells likely shared genes and resources more freely than today.
If so, today’s viruses and streamlined symbionts may echo an ancient lifestyle rather than representing biological outliers.
Sukunaarchaeum may not be alone
Scientists plan to investigate whether similar organisms exist in other marine ecosystems or symbiotic relationships.
These searches may involve reanalyzing existing metagenomic databases that could contain overlooked sequences resembling Sukunaarchaeum.
Another goal is to identify the specific host that enables Sukunaarchaeum to survive.
Without knowing its exact partner, researchers can’t fully explain how the symbiosis functions or what evolutionary pressures shaped such an extreme dependency.
The study is published in bioRxiv.
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–
Source link



