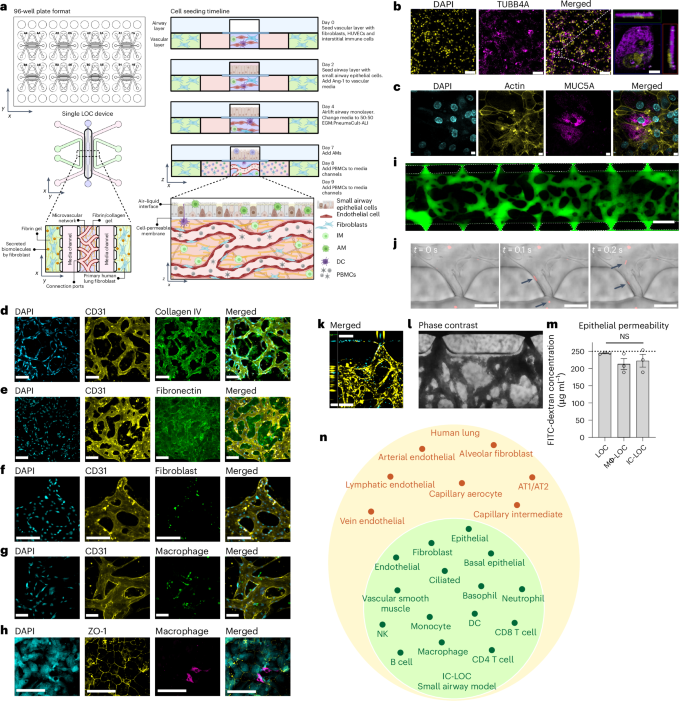
Immune-competent LOC mimics small airway cell composition
The immune-competent LOC model of the small airway comprises a three-dimensional (3D), perfusable microvascular network underneath a mature, differentiated airway epithelium at an air–liquid interface (ALI). The 3D microvascular network provides a unique advantage in that it is comprised of both endothelial cells and fibroblasts in a hydrogel matrix, in which interstitial immune cells can be incorporated. The perfusable microvascular network introduces circulating immune cells, which have the potential to migrate into the vascular network, extravasate and transmigrate in response to a stimulated epithelium. We modified our recently reported microphysiological human LOC device34 to include multiple tissue-resident and circulatory immune cell types (Fig. 1a and Supplementary Fig. 1). The modified device incorporates a monolayer of small airway epithelial cells (SAECs) at ALI separated by a cell-permeable 5.0 µm pore size polyester track etched (PETE) membrane from a 3D perfusable microvascular network to allow for immune cell migration between the two compartments. The membrane pore size was optimized to allow for active immune cell migration across the membrane in response to a chemoattractant (Supplementary Fig. 2a). A schematic of the device design, as well as the cell seeding timeline for the device, is shown in Fig. 1a. To validate that the 5.0 µm pore size membrane could support the formation of a mature, differentiated airway epithelium in the LOC device, we evaluated the expression of ciliated cell marker beta-tubulin (TUBB4A) (Fig. 1b), as well as goblet cell marker mucin 5AC (MUC5AC, Fig. 1c), indicative of a differentiated epithelium in the airway layer of the LOC. As expected, we observed cilia to be situated on the top surface of the epithelial cells within the airway layer of the lung chip devices (Fig. 1b, inset). To investigate the formation of the interstitial layer underlying the airway epithelium, we performed confocal imaging and observed networks of CD31+ human umbilical vein endothelial cells (HUVECs) (Fig. 1d,e). ECM staining showed high levels of secreted collagen IV forming a basement-like membrane around the vessels (CD31) (Fig. 1d) as well as diffuse fibronectin in the interstitial regions between the vessels (Fig. 1e). These observations align with what has been shown in the human lung, with collagen IV primarily found in basement membranes and fibronectin largely existing in the interstitial matrix35,36,37. Confocal imaging further confirmed the presence of the interstitial fibroblasts interspersed within the CD31+ vascular network (Fig. 1f). These fibroblasts are perivascular and function as stromal support, with a distinct role from those in the outer channels where the fibroblasts support the formation of vasculature through the secretion of growth factors.
a, Schematic of the 96-well plate format of the LOC device, showing the 2 × 4 grid formatted to fit on a bottomless 96-well plate, including a zoomed-in view of a single device and the cellular composition of each channel and the device seeding timeline, created using BioRender (https://BioRender.com/7wk1dzr). b,c, Representative images of the airway epithelium in the LOC device, showing DAPI in yellow and ciliated cell marker TUBB4A in magenta (scale bars, 100 µm), with inset showing TUBB4A signal situated at the top surface of the cell (scale bar, 10 µm) (b), as well as goblet cell marker Mucin5AC (magenta) and actin (yellow) (c). Scale bars, 11 µm. d, ECM staining of collagen IV (green) surrounding the formed vessels (CD31, yellow). Scale bars, 160 µm. e, Additional ECM staining of fibronectin (green) in the interstitial space along with CD31 (yellow) staining of the vessels. Scale bars, 160 µm. f, Incorporation of labelled interstitial fibroblasts (green) with vasculature (CD31, yellow). Scale bars, 160 µm. g, Incorporation of IMs (green) with vasculature (CD31, yellow). Scale bars, 80 µm. h, Representative images of the epithelium (ZO-1, yellow) with AMs (CD68, magenta). Scale bars, 100 µm. i, The microvascular network perfused with 70 kDa FITC-dextran. Scale bar, 500 µm. j, Timeframe images (Δt = 100 ms) of labelled PBMCs (red, arrows) entering the microvascular network through open lumens between ports of the device. k, Labelled PBMCs (cyan) perfused through the microvascular network (yellow), showing PBMCs entering through an open lumen. Scale bar, x-direction 160 µm, z-direction 50 µm. l, Bright-field image of whole blood perfused through the microvasculature. Scale bar, 160 µm. m, 10 kDa FITC-dextran permeability of the epithelium in LOC, Mϕ-LOC and IC-LOC devices, quantified by the airway concentration of FITC-dextran at the assay endpoint (2 h incubation). The dotted line represents the starting concentration of 250 μg ml−1. N = 3 independent devices, with statistical significance determined via ordinary one-way ANOVA followed by Tukey’s multiple comparisons test. NS, not signficant. Data are presented as mean values ± s.d. n, A graph depicting the major cell types observed in the IC-LOC and the human lung, as determined via scRNA-seq.
To determine the role of AMs and IMs on lung structure and function, we incorporated blood monocyte-derived macrophages into the LOC, denoted Mϕ-LOC. Monocytes were isolated from whole blood and differentiated with macrophage colony-stimulating factor (M-CSF) for at least 7 days before incorporation within the LOC. Media composition was optimized, and IMs were successfully incorporated into the interstitial vascular layer on day 0 at a ratio of 1:10 with the HUVECs, as shown in Fig. 1g and Supplementary Fig. 2b. By pre-labelling macrophages with CellTracker Green, we found that the IMs primarily localized perivascularly (Fig. 1g). On day 7, after establishing ALI, macrophages were also incorporated into the airway layer of the Mϕ-LOC at a concentration of 1 million cells per ml. These AMs were successfully maintained in the epithelium until at least day 12 without disrupting the integrity of the epithelial monolayer, as demonstrated by intact ZO-1 staining near CD68+ macrophages (Fig. 1h).
Furthermore, we found that macrophages adopted distinct phenotypes depending upon their location, interstitial or airway, within the device. Utilizing the UMAP and FlowSOM plugins in FlowJo version 10.8 to analyse flow cytometry data of the expression of markers CD11b, CD11c, CD206, HLA-DR, MARCO, CCR7 and CD103, we observed that day 0 (pre-device) macrophages, IMs from devices and AMs from devices each clustered separately (Extended Data Fig. 1a–c). To accurately identify IMs and AMs from the devices, IMs were pre-labelled with CellTracker Green, and AMs were pre-labelled with CellTrace Yellow before incorporation into the device. Before culture within the device, the macrophage population was largely homogeneous, with high levels of CD206 and CD11b (Extended Data Fig. 1d). Meanwhile, the IMs and AMs clustered into five separate clusters. While there was some overlap between the airway and interstitial populations, as expected, the IMs and AMs largely clustered separately and demonstrated a high level of heterogeneity after culture within the device (Extended Data Fig. 1d). The IMs retained relatively low expression of markers MARCO, CCR7, CD206 and CD103. By contrast, the AM populations expressed high levels of these markers, depending upon cluster. Furthermore, we observed an overall increase in CD11c and HLA-DR expression on AMs compared with those in pre-device macrophages (day 0). MARCO, CD11c and HLA-DR represent important mediators of cell adhesion, phagocytosis and antigen presentation in AM populations. Thus, their increase upon culture within the Mϕ-LOC demonstrates the physiologically relevant influence of the Mϕ-LOC microenvironment on cellular phenotype19,38,39,40,41,42. Thus, both IM and AM populations can be represented within the LOC, which is herein referred to as the Mϕ-LOC.
We then built on the Mϕ-LOC to include additional immune populations to enhance the physiological relevance of the device. Specifically, within the interstitial compartment, we incorporated interstitial DCs in a similar manner to IMs, at a ratio of 1:10 with the HUVECs. DCs were differentiated from blood monocytes using IL-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF) and were donor-matched to the incorporated macrophages. Lung DCs are critical in the response to foreign pathogens and the initiation of the adaptive immune response43. Here we found that the day 0 (pre-device) DC population was largely homogeneous and clustered separately from the more heterogeneous post-device DCs (Extended Data Fig. 2a–c). Before culture within the device, the DC population expressed high levels of CD206, CD11b and CD11c and clustered into a single population. However, after culture within the device, we observed an increase in heterogeneity and an increase in CD103, CCR7 and MARCO expression (Extended Data Fig. 2d). CD103+ DCs are canonically associated with the lung epithelium and are critical for the antiviral T cell response, and thus it is encouraging that we can recapitulate this phenotype within the chip44.
The severity of influenza is dependent on the host’s both innate and adaptive immunity45. Thus, to mimic this, we aimed to incorporate circulatory immune cells into the model. To this end, we first evaluated the timeline of perfusability of the vascular network within the lung chip. We observed the formation of a fully perfusable network within the device by day 9 as observed by perfusion of 70 kDa FITC-dextran (Fig. 1i and Extended Data Fig. 3a). To address the reliability and repeatability of the perfusable network formation, we characterized the dextran perfusability of devices from 8 independent experiments, ranging from 24 to 48 devices per experiment. For this analysis, we characterized devices as either ‘not perfusable’ (0/7 open ports), ‘partially perfusable’ (>0/7 but <6/7 open ports) or ‘fully perfusable’ (≥6/7 open ports). Through this, we found that across 8 independent experiments, 80.4% of devices (152 devices) were fully perfusable, 13.2% (25 devices) were partially perfusable, and 6.3% (12 devices) were not perfusable. Across the 8 experiments, the percentage of fully perfusable devices ranged from 70.3% to 94.4% (Extended Data Fig. 3b,c). After confirming the perfusable vasculature, on day 9, we added PBMCs or red blood cell (RBC)-depleted whole blood to the media channels to perfuse through the microvascular network (Fig. 1j–l). We observed the entrance of circulatory immune cells (PBMCs; Fig. 1j,k) or whole blood (Fig. 1l) into the central channel through the open lumens of the microvasculature. All immune cells (macrophages, DCs and PBMCs) used in the model were obtained from the same single donor to mitigate non-self-immune reactions as much as possible without a fully autologous system and to ensure reproducibility by avoiding donor-dependent variances. The LOC with incorporated tissue-resident (AM, IM and DC) and circulatory immune cells was coined the immune-competent LOC (IC-LOC).
The use of RBC-depleted whole blood markedly deviates from current approaches that use only PBMCs, thereby excluding the polymorphonuclear fraction of white blood cells, key mediators of immune responses. Especially in the context of viral infection, where neutrophils and other granulocytes are key responders, incorporating these cells is critical. The circulating immune populations within the devices were maintained for up to 72 h with viability of >90% in the circulating fraction and >75% in the extravasated interstitial fraction (Supplementary Fig. 3). In addition, we did not observe any substantial activation of the T cells owing to culture within the device, as evidenced by very low levels of activation markers CD25, CD69, HLA-DR, granzyme B, interferon (IFN)-γ, NKp44 and NKG2D after 72 h within the device, comparable to that of the day 0 (pre-device) PBMCs (Extended Data Fig. 4a). However, we did observe mild activation of NK cells upon 72 h culture within the chip, with an increase in select activation markers such as CD25, CD69 and NKp44 but a decrease in others such as HLA-DR, granzyme B and NKG2D (Extended Data Fig. 4b).
To examine the integrity of the airway barrier in the presence of immune cells, we performed an FITC-dextran permeability assay by adding 250 μg ml−1 of 10 kDa FITC-dextran in PBS to the airway chamber. We observed that the concentration of the FITC-dextran did not significantly change after 2 h of incubation, irrespective of LOC, Mϕ-LOC and IC-LOC (Fig. 1m), indicating the formation of a tight epithelial barrier. The choice of 10 kDa dextran was based on previous publications46. In addition, we observed no significant changes in the vasculature characteristics between the three models (LOC, Mϕ-LOC and IC-LOC) (Supplementary Fig. 4).
To further understand the cellular heterogeneity of the IC-LOC system, we performed scRNA-seq on devices with incorporated AMs, IMs, DCs and RBC-depleted whole blood and analysed the resulting transcriptomic data using the Seurat package in R47,48,49,50. Through scRNA-seq analysis of our IC-LOC and a published human lung atlas scRNA-seq dataset51, we found that we were able to recapitulate the majority of the cell types found in the in vivo lung that correspond to those of the small airway (Fig. 1n and Supplementary Fig. 5), as our focus was specifically on the small airway in the current study. Specifically, when evaluating the resulting clusters from the integrated dataset, we found that the majority were shared between the IC-LOC and the human lung, including T cell, B cell, NK cell, DC, macrophage, monocyte, neutrophil, epithelial cell, fibroblast and endothelial cell clusters. As expected, the cell types that were exclusively found in the in vivo human lung dataset corresponded primarily to alveolar-associated cells, including alveolar epithelial cells, alveolar fibroblasts, lymphatic endothelial cells, and specialized arterial and vein endothelial cells. When considering the overall comparison, we found that the IC-LOC recapitulates the cell types expected in the in vivo small airways in human lungs. While most cell types were recapitulated, we observe differences in proportions relative to the human lung dataset (Supplementary Fig. 5). This may be explained by the incorporation of both the central channel (interstitium and airway) and circulatory cells from the media channels in our IC-LOC dataset. By contrast, the human lung dataset would differ in the proportion of blood cells and includes regions of the lung outside of the small airways. Furthermore, while the overarching cell types are represented, more in-depth sequencing analysis is required to determine whether phenotypic subpopulations are maintained. Regardless, we find that our IC-LOC models are among the most complete in vitro representations of the small airway regions of the human lung thus far.
Establishing severe influenza in the LOC
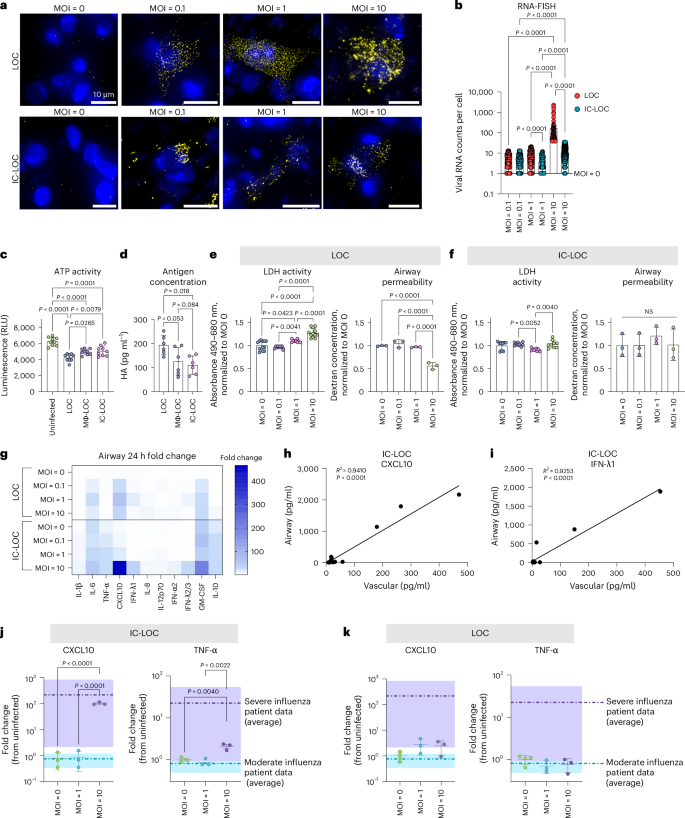
To develop a severe influenza model within the LOC, the epithelium of LOC, Mϕ-LOC and IC-LOC devices were infected with live H1N1 (A/PR/08/34). We evaluated a series of MOIs ranging from 0.1 to 10 to determine the MOI that best recapitulated select aspects of severe influenza. Infection was started on day 9, after establishing epithelial ALI (day 4) and introducing AMs (day 7) with circulatory immune cell populations (day 9). We utilized single-molecule RNA fluorescence in situ hybridization (RNA-FISH) imaging to detect and quantify intracellular viral RNA levels at the single cell level at 72 h post-infection (hpi) in the LOC and IC-LOC at varying MOIs (Fig. 2a). We observed a significant spike in viral RNA counts per cell at MOI 10 in both LOC and IC-LOC devices, averaging nearly 100 viral RNA counts per cell in the LOC epithelium, with IC-LOC resulting in comparatively lower viral loads (Fig. 2b).
a, Representative RNA-FISH images of the epithelial layer of the device, showing nuclei in blue and viral H1N1 RNA in yellow for the LOC and IC-LOC at MOI = 0, 0.1, 1 and 10. b, Quantification of the viral RNA counts per cell, as determined via RNA-FISH. N = 3 independent devices. Statistical significance determined via one-way ANOVA with Kruskal–Wallis test, and P values are shown. c, Relative quantification of infectious viral titre via a ViralTox Glo assay. Relative luminescence (RLU) corresponds to ATP activity of H1N1-susceptible MDCK cells cultured with the cell lysis of uninfected devices or LOC, Mϕ-LOC or IC-LOC devices infected at MOI = 10. N = 10 independent devices. d, Quantification of antigen (HA) concentration in the cell lysis of LOC, Mϕ-LOC or IC-LOC devices infected at MOI = 10. N = 6 independent devices. e, Quantification of epithelial damage via LDH activity and airway permeability in the LOC in response to H1N1 infection at MOI. N = 8 independent devices. f, Quantification of epithelial damage via LDH activity and airway permeability in the IC-LOC in response to H1N1 infection at varying MOIs. N = 3 independent devices. g, LEGENDplex analysis of the cytokine release profiles in the LOC and IC-LOC 24 hpi at varying MOIs. Heat map shows the fold change from time zero (pre-infection). N = 4 independent devices. h,i, Simple linear regression correlation of airway and vascular levels of CXCL10 (h) and IFN-λ1 (i) in the IC-LOC. N = 14 independent devices. j,k, Comparison of cytokine release profiles in the airway layer of the IC-LOC (j) and LOC (k) to that of BAL fluid from patients with moderate and severe influenza. Fold change from uninfected/healthy is shown. Purple bands denote the range of patient data with severe influenza, while teal bands denote the range of patients with moderate influenza. N = 3 independent devices. All immune-competent devices use cells from the same immune cell donor. All statistical significance determined via one-way ANOVA followed by Tukey’s multiple comparisons test unless otherwise noted. Data are presented as mean values ± s.d.
To complement the RNA-FISH observations, we further quantified viral levels in devices infected at MOI 10 using a Viral ToxGlo assay (Promega) and hemagglutinin-specific (HA) enzyme-linked immunosorbent assays (ELISAs) (Fig. 2c,d). The Viral ToxGlo assay involved culturing H1N1-susceptible Madin–Darby canine kidney (MDCK) cells with the collected media and cell lysis from infected devices, thus pooling all intra- and extracellular virions from an infected device. In this assay, decreased ATP activity corresponds to increased viral-induced cell death52. We observed significantly more viral-induced cell death from LOC samples than Mϕ-LOC and IC-LOC samples, indicating a higher titre of infectious virus in the LOC devices (Fig. 2c). Antigen (HA) concentration was also measured in the cell lysis of infected devices via ELISA to quantify intracellular viral antigens. Here we also observed a significantly higher antigen concentration in the LOC compared with the Mϕ-LOC and IC-LOC samples (Fig. 2d).
In addition, we evaluated epithelial damage in the LOC and IC-LOC at varying MOIs via lactate dehydrogenase (LDH) activity and epithelial permeability assays. We observed increased LDH activity with increasing MOI in the LOC devices and a significant increase in epithelial permeability in the LOC at MOI 10, indicative of structural damage to the epithelium (Fig. 2e). This damage was largely prevented in the IC-LOC devices (Fig. 2f). To further quantify the permeability, we took an alternative approach to previous reported methods of apparent permeability calculations. Apparent permeability coefficient (Papp) values are highly context dependent and, in LOC devices, can differ based on the design and material properties of the devices, as well as the experimental conditions22. Furthermore, Papp values are typically calculated using concentration measurements from the basolateral side of the membrane. While this is feasible in more canonical bilayer LOC models, the hydrogel underlying our airway epithelium makes taking direct measurements from the basolateral compartment technically challenging. While samples can be taken from the adjacent media channels, this involves diffusion through the hydrogel along the y-axis in addition to the diffusion from the airway chamber along the z-axis. Because of this, these measurements do not accurately reflect the airway permeability, and as such, permeability was purposefully presented as the dextran concentration in the airway compartment, as reported elsewhere20. However, for ease of comparison to other existing models, we have estimated apparent permeability values by assuming conservation of mass to estimate the amount of dextran that passed across the membrane into the basolateral compartment (Extended Data Fig. 5). Our results show the log[Papp] for uninfected control devices to be around −6 in both the LOC (−6.53) and IC-LOC (−5.93) (Extended Data Fig. 5). In a bilayer LOC model, Si et al.18 also reported the apparent permeability (log[Papp]) in a human airway chip with no infection to be approximately −6. In our LOC, 72 h of severe infection with H1N1 virus significantly reduced the log[Papp] from −6.53 to −5.20. In the IC-LOC, log[Papp] was not significantly affected, only decreasing from −5.93 to −5.84, indicating that epithelial barrier permeability was minimally compromised in the IC-LOC. Taken together, the viral quantification and epithelial damage assays confirm the viral titres and infection and elucidate the protective role of the immune cells in the Mϕ-LOC and IC-LOC devices.
We further observed that MOI 10 was necessary to induce high levels of cytokine secretion associated with severe influenza, often referred to as a cytokine storm, with significant spikes in IL-1β, IL-8, TNF-α, CXCL10, IFN-λ1, IFN-λ2/3, IFN-α2, GM-CSF and IL-10 at 72 hpi ranging from 5- to 400-fold increase from time zero (pre-infection) (Fig. 2g). Previous reports indicate that CXCL10 levels in the BAL and plasma uniquely correlate in patients with severe influenza and SARS-CoV-2, with plasma levels of CXCL10 even predicting patient outcomes in a study performed by Reynolds et al.53. Here we aimed to illustrate that our system mirrors in vivo observations by detecting CXCL10 as a key cytokine whose elevated levels on the vascular side of the device correlate with elevations on the airway side. We observed that the CXCL10 levels in the airway and vascular regions of the infected IC-LOC device uniquely correlated, while 11 of the additional evaluated cytokines did not (Fig. 2h and Supplementary Fig. 6). Notably, these correlations were absent in the LOC or Mϕ-LOC models (Supplementary Figs. 7 and 8). Furthermore, we discovered that in the IC-LOC, the airway and vascular levels of IFN-λ1, which were not evaluated in the patient data reported by Reynolds et al., also correlated (Fig. 2i). Obtaining cytokine measurements from the BAL fluid in patients is extremely invasive, whereas obtaining plasma samples is comparatively simple; thus, developing a comprehensive array of plasma cytokines to understand airway inflammation could be highly impactful. Here we demonstrate that our IC-LOC, but not LOC or Mϕ-LOC, can recapitulate BAL and plasma cytokine relationships observed in patients.
To further evaluate the ability of the IC-LOC to recapitulate the cytokine storm observed in vivo, we compared the fold changes in secreted cytokines CXCL10, TNF-α and IL-1β in the airway of the IC-LOC to those in the BAL fluid of patients with moderate and severe influenza infection53, compared with uninfected IC-LOC devices and healthy patients, respectively. In IC-LOC devices, MOI 10 resulted in an increase in CXCL10 and TNF-α that corresponded to the increase observed in patients with severe influenza (Fig. 2j). Meanwhile, LOC devices were unable to produce physiologically relevant increases in cytokine levels when compared with patient data, regardless of MOI (Fig. 2k). Mϕ-LOC devices only produced physiologically relevant increases in CXCL10, with no significant increase in TNF-α (Supplementary Fig. 9). Meanwhile, for IL-1β, MOI 10 elicited a significant increase only in the IC-LOC, not in LOC or Mϕ-LOC devices (Supplementary Fig. 9). These findings indicate that the IC-LOC system recapitulates the hyperinflammatory environment observed in patients with severe influenza when infected at MOI 10. Therefore, MOI 10 was used in all subsequent experiments to mimic severe influenza infection.
IC-LOC reveals critical structural changes in infected airways
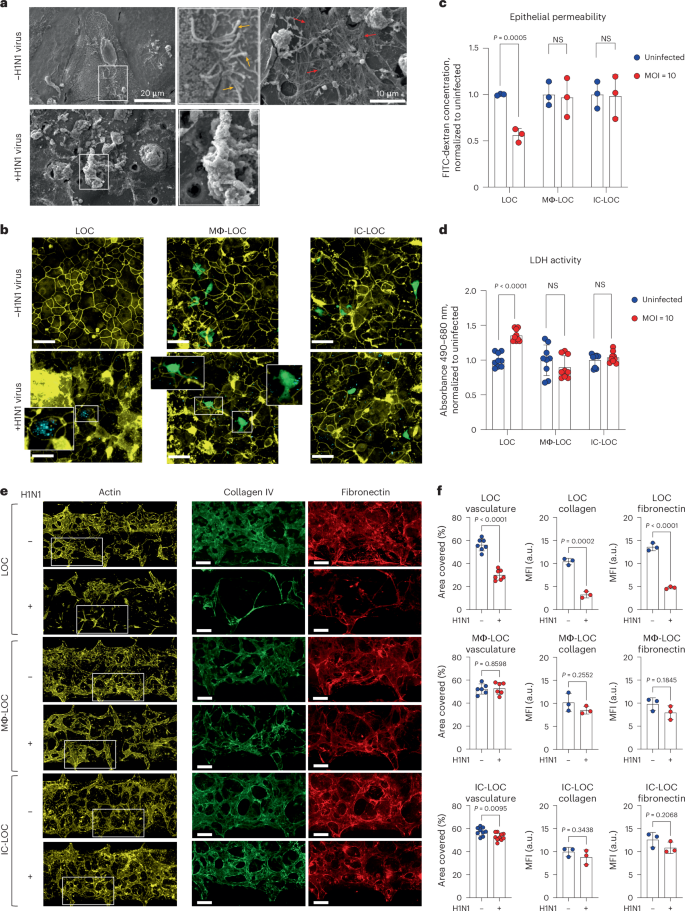
In patients, severe H1N1 infection results in a disruption of lung integrity. However, whether specific immune cell subtypes contribute to the exacerbation or prevention of damage to structural cells within the lung microenvironment remains unclear. To this end, we first evaluated structural changes in the LOC epithelium in response to severe influenza infection through scanning electron microscopy (SEM). Healthy, uninfected devices showed an intact monolayer with cilia and mucus secretions (Fig. 3a). Meanwhile, upon severe influenza infection, SEM imaging revealed substantial epithelial disruption (Fig. 3a). To further investigate the specific role of both tissue-resident and circulating immune cells on H1N1-mediated epithelial damage, we evaluated the presence of tight junction protein ZO-1 in the epithelial layer of various LOC devices 72 hpi using confocal microscopy (Fig. 3b). The LOC devices, devoid of immune cells, experienced significant epithelial ZO-1 disruption upon H1N1 infection. By contrast, the presence of immune cells in the Mϕ-LOC and IC-LOC models reduced the tight junction irregularity. High-resolution confocal microscopy further revealed active uptake of viral particles by AMs (Fig. 3b, insets). We also observed that the viral load within epithelial cells was lessened by the presence of AMs (Fig. 3b, insets). Epithelial permeability and LDH activity analysis across LOC, Mϕ-LOC and IC-LOC samples revealed that both the Mϕ-LOC and IC-LOC largely prevented the epithelial damage observed in LOC devices (Fig. 3c,d). These findings demonstrate the key role that tissue-resident and circulatory immune cells play in maintaining the integrity of the epithelial monolayer in these devices.
a, SEM of the epithelium in the LOC with and without severe influenza infection. Yellow arrows, cilia. Red arrows, mucus. b, Epithelial H1N1-mediated damage in the LOC, Mϕ-LOC and IC-LOC shown by irregularity in ZO-1 (yellow) staining, with H1N1 shown in cyan and AMs labelled with CellTrace Green (green). c, Quantification of epithelial barrier function via epithelial permeability assay with 10 kDa FITC-dextran. The graph shows FITC-dextran concentration in the airway channel after 2 h incubation, normalized to uninfected controls. N = 3 independent devices. d, Quantification of epithelial damage via LDH activity as determined via a colorimetric assay. Absorbance values shown normalized to uninfected controls. N = 8 independent devices. e, Representative images of the ECM in LOC, Mϕ-LOC and IC-LOC in severely infected and uninfected devices. Images show the vascular network (actin, yellow) and related ECM proteins (collagen, green; fibronectin, red). f, Quantification of the percent area covered by the vasculature in the LOC, Mϕ-LOC and IC-LOC. In addition, quantification of the collagen and fibronectin levels, as quantified by MFI, in each device in response to infection are also shown. N = 7 independent devices for vasculature analysis and N = 3 independent devices for collagen and fibronectin analysis. Scale bar for all images, 160 µm. All immune-competent devices use immune cell from the same immune cell donor. Statistics represent two-tailed unpaired t-tests, with P value shown on each graph. Data are presented as mean values ± s.d.
In addition to characterizing the effect of immune cell incorporation and H1N1 infection on the epithelium, we also investigated their effect on the vascular network. Using AngioTool (version 0.5), we quantified the vascular percent area coverage and average vessel length. We observed that in the LOC, H1N1 infection destroyed the vascular network, as can be qualitatively observed in the representative images shown in Fig. 3e. Quantitative analysis revealed that H1N1 infection in the LOC significantly decreased vasculature percent area coverage and average vessel length (Fig. 3f and Supplementary Fig. 10). However, tissue-resident macrophages in the Mϕ-LOC mitigated the observed vascular damage. With the presence of macrophages, H1N1 infection had no significant effect on vascular percent area covered or average vessel length. While the overall vascular network of the IC-LOC was largely maintained in the severe H1N1 condition, the infected IC-LOC showed few areas of vascular disruption as evident with an overall modest but significant decrease in the percent area covered compared with the uninfected devices (Fig. 3f). This is unsurprising, as severe influenza infection is known to cause vascular disruption through the high levels of secreted cytokines54. Nevertheless, as with the airway layer of the device, incorporating tissue-resident immune cells in the Mϕ-LOC and IC-LOC can protect the vascular network from undergoing significant structural damage upon severe infection. ECM remodelling also occurs in response to lung respiratory infections55. These changes include increased deposition of ECM fragments and collagen, and massive formation and deposition of fibronectin, among others. However, these observations are largely made from patients with SARS-CoV-255. In mice, SARS-CoV-2 infection increased collagen deposition in the lungs compared with influenza-infected mice. It remains unclear how the immune competency of lung tissues impacts alterations to the ECM in response to severe H1N1 infection. Thus, to investigate the impact of immune cells on the extracellular environment of the lung in severe H1N1, we quantified the effect of H1N1 infection on secreted ECM proteins. In the immune-deficient LOC, severe H1N1 infection significantly reduced collagen IV and fibronectin deposition in the interstitium (Fig. 3e,f). Specifically, the percent area covered by collagen IV was significantly lower in LOC devices with severe H1N1 infection, indicating that fewer cells were secreting this ECM protein, even after normalization to the actin percent area covered to account for differences in total cells present. Similar to collagen IV, fibronectin secretion levels were also lower in H1N1-infected LOC devices, indicating a change in the interstitial ECM composition. The mean fluorescence intensity (MFI) was significantly lower in response to H1N1 infection, indicating that the cells were producing lower levels of these ECM proteins (Fig. 3f). By contrast, in the Mϕ-LOC and IC-LOC devices, collagen IV and fibronectin levels were modestly but insignificantly reduced, therefore suggesting that the local niche was largely maintained. With this, we find that the lack of immune components may result in an overstated impact of viral infection in vitro, as immune competency appears to significantly protect from viral-induced structural damage.
IC-LOC demonstrates immune cell influx and viral uptake
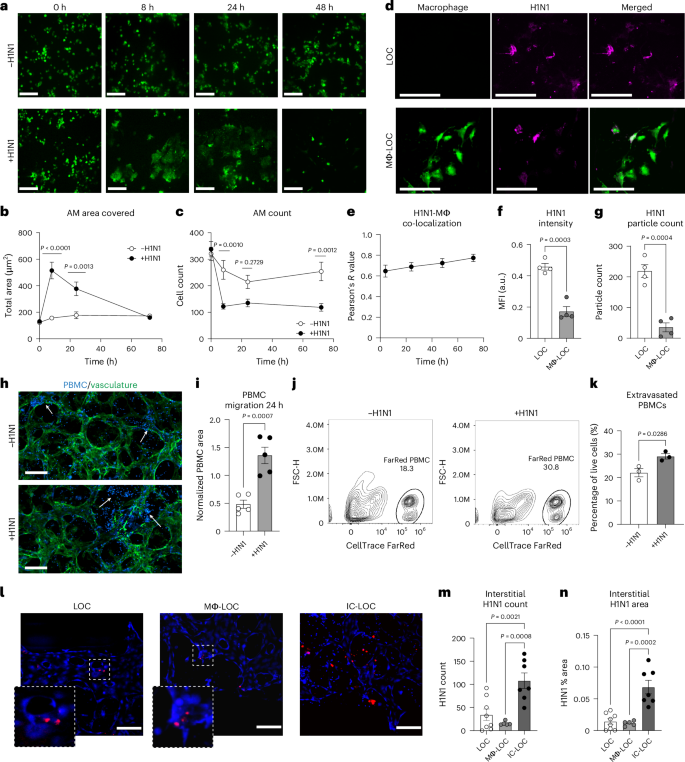
To further understand the role of AMs in the progression of H1N1 infection, we labelled macrophages with CellTracker Green and imaged the airway epithelium periodically over the 72 h infection period (Fig. 4a). We found that at 8 hpi, the AMs in the infected devices increased dramatically in size in response to infection (Fig. 4b), a phenomenon that has not been previously reported. The overall number of AMs also plummeted after 8 h in the infected devices but remained relatively stable in uninfected devices (Fig. 4c). Furthermore, it was observed that the AMs co-localized with PKH26 fluorescent cell linker dye-labelled H1N1 viral particles as shown by a high Pearson’s R value, determined using ImageJ (version 1.54) co-localization analysis, ranging from 0.64 to 0.79 and increasing over time (Fig. 4d,e). At the 72 h endpoint, both the MFI of the viral signal and the absolute number of observable viral particles in the epithelium were higher in devices without macrophages (Fig. 4f,g). This, along with the co-localization, suggests that the AMs uptake the virus upon initial infection and protect the lung from the progression of infection.
a, Representative images of the change in AM size, shape and abundance over time in the Mϕ-LOC device with and without severe influenza infection (MOI = 10). Scale bars, 160 µm. b, Quantification of the number of AMs observed in devices with and without severe infection. N = 10 independent devices. c, Quantification of the total area covered by AMs in devices with and without severe infection. N = 10 independent devices. d, AM (green) co-localization with PKH26 dye-labelled H1N1 viral particles (magenta). Scale bars, 160 µm. e, Co-localization of AMs with labelled H1N1 particles. N = 7 independent devices. f,g, MFI (f) and particle count (g) of the labelled H1N1 signal in LOC devices with and without tissue-resident macrophages. N = 4 independent devices. h, PBMC infiltration into the interstitial layer of the IC-LOC device, with and without severe influenza infection (vasculature, green; pre-labelled PBMCs, blue). Scale bars, 160 µm. i, Quantification of labelled PBMC infiltration into the interstitial layer at 24 hpi. N = 5 independent devices. j, Representative flow cytometry analysis plots of the CellTrace FarRed labelled PBMC population from the central channel of the IC-LOC. k, Percentage of live cells from the central channel that are PBMCs (CellTrace FarRed+) in uninfected and infected IC-LOC devices. N = 3 independent samples, with each sample = 4 pooled devices. l, Representative images showing viral nucleoprotein (H1N1 NP, red) staining in the vascular layer (DAPI, blue). Scale bars, 150 µm. m,n, Quantification of interstitial H1N1 particle count (m) and area (n), as determined by H1N1 nucleoprotein staining in LOC, Mϕ-LOC and IC-LOC devices, determined via ordinary one-way ANOVA followed by Tukey’s multiple comparisons test. N = 7 independent devices. All immune-competent devices use immune cells from the same immune cell donor. All statistical significance determined via two-tailed unpaired t-test unless otherwise noted. Data are presented as mean values ± s.d.
The recruitment of circulating immune cells from the vascular space into infected tissue is central to the lung pathology in influenza infection25,56. However, the most relevant previous work using a human airway chip and influenza infection in an in vitro model has used only primary neutrophils. A major caveat of previous systems is that they lack the interstitial space and chemokine gradients generated across these various epithelial and endothelial barriers, including basement membranes, during viral infections. However, in influenza infection in patients, the collagen-rich interstitium plays a vital role where immune cells such as neutrophils and T cells navigate through the interstitium, guided by chemokines to be recruited to the airway space57. To overcome this technical barrier and knowledge gap, we characterized the effect of H1N1 infection on PBMC migration in the IC-LOC by labelling PBMCs with CellTrace Violet and perfusing them through the microvascular network (Fig. 4h,i). We observed a significant increase in migration of PBMCs from the media channels into the central channel of the device in response to H1N1 infection, as measured by the percent area covered by the labelled PBMCs at 24 hpi, normalized to the area covered by PBMCs at time zero to account strictly for migration into the central channel in response to infection (Fig. 4i). Importantly, we also observed a qualitative increase in PBMCs within the central channel that had extravasated from the vessels into the interstitial space in the infected devices. By contrast, in uninfected devices, the PBMCs within the central channel primarily remained within the microvascular network (Fig. 4h, arrows, and Extended Data Fig. 6). This qualitative observation was further validated through flow cytometric analysis of the central channel of IC-LOC devices with perfused PBMCs labelled with CellTracker FarRed. IC-LOC devices were flushed with PBS to remove any free PBMCs, and then the central channel hydrogel was degraded, collected and evaluated via flow cytometry. We observed a significant increase in the percentage of extravasated PBMCs present in the central channel of infected devices (Fig. 4j,k). Thus, we not only observed an increase in PBMC infiltration from the media channels into the vascular network, but also we observed an increase in PBMCs which extravasated into the interstitial space in the presence of an H1N1-infected epithelium, reminiscent of the immune cell infiltration and recruitment observed in severe H1N1 in vivo. To the best of our knowledge, our work is the first demonstration of the recruitment of PBMCs through an endothelial network and into interstitial space in an in vitro lung infection model.
Arguably, the recruitment of PBMCs from the vascular network to the interstitium could be due to virus infiltration in the interstitial space. Using confocal microscopy, we probed the interstitium of infected IC-LOC devices for the influenza A nucleoprotein (NP) (Fig. 4l–n). Indeed, we observed a substantial viral signal in the interstitium of the IC-LOC in both observable particle count and percent area covered. However, HUVECs are relatively resistant to direct influenza infection, and, accordingly, minimal viral signal was detected in the interstitial layer of the LOC and Mϕ-LOC devices (Fig. 4m,n). This suggests that interstitial DCs and extravasation of circulating immune populations may be necessary for viral trafficking from the epithelium.
Severe infection in IC-LOC induces systemic cytokine storm
In vivo, severe H1N1 infection is characterized by significantly elevated levels of inflammatory cytokines, including but not limited to IFNs, IL-1, IL-6 and IL-10 in both the BAL fluid and serum53,58,59,60. In recent years, high systemic levels of cytokines and their association with disease severity have frequently been referred to as a ‘cytokine storm’. However, it remains unclear whether specific inflammatory molecules drive lung pathology or serve as correlates of other tissue-level mechanisms. To study this, we characterized the cytokine response to severe H1N1 infection from samples collected from both the airway and interstitial layers of infected and uninfected LOC, Mϕ-LOC and IC-LOC devices at various timepoints, including 0, 2, 24 and 72 hpi.
In the LOC, only a modest cytokine response was observed in response to infection (Fig. 5a,b and Supplementary Figs. 11 and 12), with IFN-λ2/3 being the most highly secreted cytokine. This is expected as IFN-λ is secreted primarily by epithelial cells in response to infection61. The Mϕ-LOC produced a notable increase in inflammatory cytokines in the airway layer, with elevated levels of IL-10, IL-1β, GM-CSF and CXCL10 in response to infection, but failed to generate a significant interstitial response (Fig. 5a,b and Supplementary Figs. 11 and 12). However, in the IC-LOC, we observed a considerable cytokine response generated in both the airway and the interstitial layers with a significant increase in IL-10, IL-1β, GM-CSF, IFN-λ1, CXCL10, IFN-λ2/3, IL-6 and IFNγ. These findings, when coupled with the lack of detectable viral signal in the endothelium of infected LOC and Mϕ-LOC devices, indicate that full immunocompetency results in a broad spectrum increase in cytokines and other inflammatory proteins, which could potentially have a damaging impact on the lung endothelium structure.
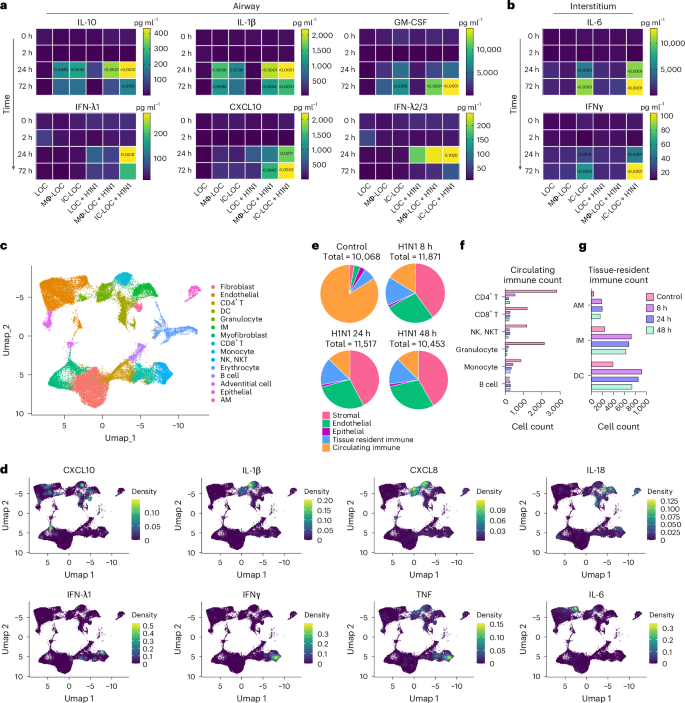
a,b, Secreted pro-inflammatory cytokine levels in the epithelial airway (a) and interstitial vascular (b) layers of the device in healthy and infected (H1N1, MOI = 10) conditions, statistical significance compared with time 0 h determined via ordinary one-way ANOVA with P values shown on the charts. N = 6–7 independent devices from the same immune cell donor across at least 2 independent experiments. c, UMAP visualization of the integrated datasets (control, 8 h, 24 h and 48 h) identifying 15 distinct cell types within the IC-LOC. d, Visualization of select key cytokine expression patterns in UMAP space using the kernel density estimation method in the R package Nebulosa. e, Pie charts depicting the relative proportions of stromal, endothelial, epithelial, tissue-resident immune and circulating immune cells identified in the scRNA-seq data. f,g, Raw counts of circulating (f) and tissue-resident (g) immune cells from the scRNA-seq data.
Through scRNA-seq analysis of both healthy and infected IC-LOC devices, we could also identify several key producers of inflammatory cytokines. Specifically, we compared samples from control uninfected devices and devices that had been infected with H1N1 for 8 h, 24 h and 48 h timepoints and analysed the resulting transcriptomic data using the Seurat package in R47,48,49,50. To ensure that any observed effects were not due to differences in the length of device culture, all devices were cultured for the same amount of time, and viral infection was initiated at 8 h, 24 h or 48 h before the experiment endpoint, with control devices cultured for the full time with no infection. Here we utilized RBC-depleted whole blood to incorporate both leukocytes and granulocytes in the circulating immune populations, in addition to the tissue-resident IMs, DCs and AMs. Cells were combined from both layers of the IC-LOC devices for sequencing, and gene expression profiles were compared with known datasets and used to annotate cell type and subset62. Through scRNA-seq analysis, we were able to detect 15 distinct cell types, including endothelial cells, fibroblasts, myofibroblasts, adventitial cells, epithelial cells, IMs, AMs, DCs, monocytes, granulocytes, CD4 T cells, CD8 T cells, NK/NKT cells, B cells and erythrocytes, the last of which were excluded during analysis (Fig. 5c and Extended Data Fig. 7).
We then probed for inflammatory cytokines to identify key players in the observed cytokine storm. To this end, we found that monocytes were largely responsible for the production of IL-10 and IL-1β, IMs were the main producers of IL-18, granulocytes produced significant levels of IL-8, and NK/NKT and T cells were largely responsible for IFNγ, IFN-λ1 and TNF production (Fig. 5c,d). Notably, we found that endothelial and stromal cells produced significant amounts of IL-10, IL-12 and IL-6, aligning with the notion that lung endothelial cells are key orchestrators of cytokine amplification during influenza virus infections57. In the multiplexed cytokine analyses (Fig. 5a,b), we did not observe significant levels of these cytokines secreted in either the infected LOC or Mϕ-LOC. Thus, we hypothesize that the activation of circulating immune cells is necessary to exacerbate the activation of endothelial and stromal cells and induce the secretion of this inflammatory milieu, further underscoring the necessity of the fully immunocompetent IC-LOC to mimic the antiviral inflammatory immune response and highlighting the complex signalling cascade that occurs in severe influenza.
Severe H1N1 infection alters stromal and immune populations
Analysis of scRNA-seq data revealed an overall temporal change in cellular proportions in response to severe influenza infection, which remains poorly understood owing to a lack of controlled human influenza studies from patients with severe influenza. Specifically, the circulating immune populations (defined as all T cells, B cells, NK/NKT cells, monocytes and granulocytes) accounted for 83.9% of the cells in the uninfected control sample, compared with only 16.2%, 12.8% and 12.5% in the 8 h, 24 h and 48 h of severely infected samples, respectively (Fig. 5e). We specifically observed a substantial decrease in CD4 and CD8 T cells, NK/NKT cells and granulocytes, with a modest decrease in monocytes. Notably, the B cell population numbers remained similar or even increased in response to H1N1 infection (Fig. 5f). Meanwhile, the tissue-resident immune populations (defined as AMs, IMs and DCs) increased slightly (Fig. 5g).
We observed a notable decrease in epithelial cells, most likely in direct response to viral infection. By contrast, the percentage of stromal and endothelial cells increased significantly—likely as an artefact of cell survival as opposed to cell proliferation54,63. To confirm this, we evaluated the expression patterns of various proliferation markers, including PIF1, CDK1, UBE2C, FAM111B and TOP2A, and found that the stromal and endothelial cells did not express substantial levels of any of the evaluated proliferation markers. However, this did yield an interesting finding in which B cells within the infected devices were the most prominently proliferating cell type (Extended Data Fig. 8), in line with our previous observation of the B cell proportions in infected devices.
Severe infection boosts stromal–immune crosstalk in IC-LOC
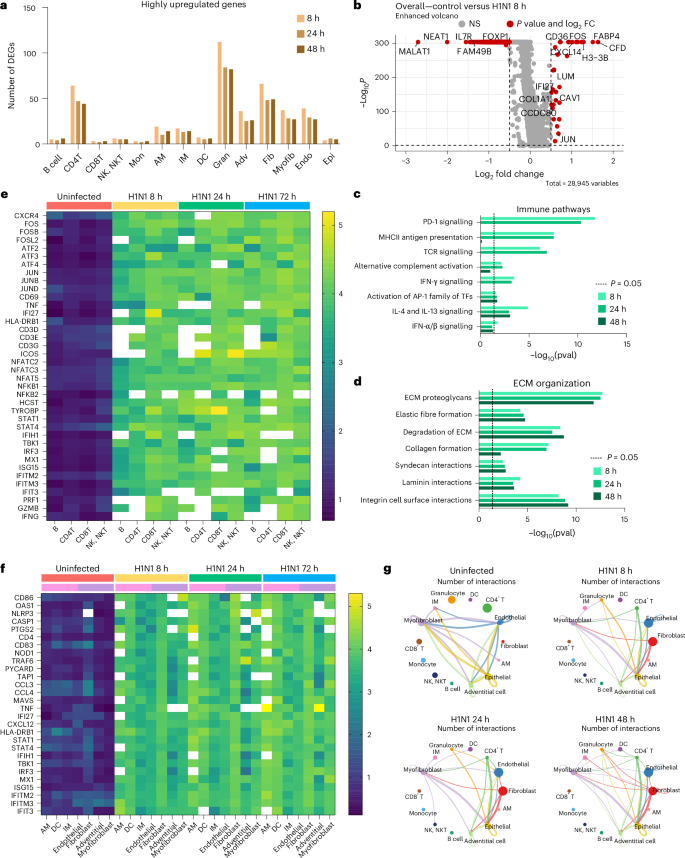
In addition to a significant variation in cellular proportions, we observed a major transcriptional shutdown in response to severe influenza infection in the IC-LOC. The median number of genes per cell in the control sample was 2,792 compared with 218, 171 and 155 in the 8 h, 24 h and 48 h infection samples, respectively. Although we observed a major transcriptional shutdown in response to severe H1N1 infection, as evidenced by the downregulation of many ribosomal genes, a small number of genes were also upregulated in response to infection (Fig. 6a and Supplementary Fig. 13). Across the 8 h, 24 h and 48 h infection samples, 215, 157 and 160 unique genes, respectively, were highly upregulated (fold change (FC) >1.5) compared with the control.
a, The number of highly upregulated genes (FC >1.5) per cell type compared with the control devices. b, Volcano plot visualization of DEG analysis, highlighting all genes significantly highly (P < 0.05, |FC| >1.5) up- or downregulated across all cell populations when comparing uninfected control to 8 h H1N1 infection IC-LOC devices. Statistical significance determined via Wilcoxon rank sum test. c,d, Pathways represented in the top 10% of upregulated genes in each infected condition, comprised primarily of immune pathways (c) and ECM interactions (d). Statistical significance determined via binomial test. e, Heat map of expression levels of inflammatory, antiviral and immune activation genes in B, CD4 T, CD8 T and NK/NKT cells from uninfected and infected devices showing increased expression levels in the infected devices. Heat map shows average expression levels of each gene for cells with non-zero expression. White boxes represent genes that are not expressed in the given cell type. f, Heat map of expression levels of innate immune response genes in AM, DC, IM, endothelial, fibroblast, adventitial and myofibroblast populations. g, Top 10% of CellChat predicted cell–cell interactions, demonstrating an increase in both stromal and immune cell interactions in response to infection. DEGs, differentially expressed genes; Mon, monocytes; Gran, granulocytes; Adv, adventitial cells; Fib, fibroblasts; Myofib, myofibroblasts; Endo, endothelial cells; Epi, epithelial cells; TFs, transcription factors; pval, P value.
When evaluating total differential gene expression levels between infected and control devices, we found that most of the largely downregulated genes (FC <−1.5) corresponded to cell cycle and transcriptional regulatory genes such as MALAT1, NEAT1 and FAM49B (Fig. 6b and Supplementary Figs. 14–16). IL7R was also significantly downregulated, as is typical following TCR engagement64. When considering genes that were upregulated in the total cell population, we identified many genes canonically involved in immune cell recruitment and response, such as CXCL14, CFD and IFI27. CD36, a scavenger receptor that responds to pathogen-associated molecular patterns and damage-associated molecular patterns, was also significantly elevated. Beyond these, ECM-related genes such as COL1A1 and CCDC80, positive regulators of ECM reorganization and cell-substrate adhesion, were also elevated.
Pathway analysis revealed that the H1N1-upregulated genes corresponded to immune response pathways, including MHC-II antigen presentation, TCR signalling and IFN signalling, as well as ECM remodelling pathways, including ECM proteoglycans, ECM degradation, laminin interactions and integrin cell surface interactions (Fig. 6c,d), as determined using Reactome pathway analysis tools65,66,67. While we did not observe significant differences in the fluorescence microscopy of secreted collagen IV and fibronectin in response to influenza infection, the transcriptomic analysis revealed that severe influenza had a substantial effect on the ECM milieu and cell–ECM interactions in the IC-LOC, beyond simple collagen and fibronectin levels (Fig. 6d). Using gene set enrichment analysis (GSEA), we identified key pathways upregulated in each cell type subset compared with the control68. Notably, some of the most upregulated pathways included the inflammatory response, IFN-α response, IFN-γ response and oxidative phosphorylation (Supplementary Fig. 17). The key cell types involved in these upregulated pathways included endothelial cells, fibroblasts, adventitial cells, epithelial cells, DCs and granulocytes. Stromal cells are heavily represented in these pathways and thus highlight the importance of including the interstitium in in vitro infection models of the lung.
While the differentially expressed genes and upregulated pathways alluded to overall changes, we next evaluated gene expression patterns across specific cell types in the IC-LOC in response to severe infection. Here we evaluated levels of key activation and antiviral response genes in the uninfected and infected (8 h, 24 h and 48 h) conditions. We first considered the adaptive immune populations within the IC-LOC, including B, CD4 T, CD8 T and NK/NKT cells (Fig. 6e). Cells were first filtered to only account for those that had non-zero expression of the target genes, and then average expression levels were calculated for each gene of interest. We probed for markers of adaptive immune cell (T/B/NK/NKT) activation (CD69, TNF, HLA-DRB1, CD3, ICOS, GZMB, PRF1 and IFNG), the AP-1 transcription factor complex (FOS, FOSB, FOSL2, ATF2-4, JUN, JUNB and JUND), NF-κB pathway (NFKB1/2), and IFN response and antiviral genes (STAT1/4, IFI27, IFIH1, IRF3, ISG15, IFITM2/3 and IFIT3). Across all evaluated genes, we saw significant increases in expression in adaptive immune cells from infected devices from as early as 8 hpi and increasing over time. We then probed the tissue-resident immune populations (AM, DC and IM) for similar activation and antiviral response genes, specifically innate immune cell activation markers (CD86, CD83 and HLA-DRB1), IFN-stimulated genes (OAS1, MX1, ISG15, IFITM2/3, IFT3, IFI27 and STAT1/4), inflammasome components (NLRP3, CASP1 and PYCARD), and cytokine and secreted signalling genes (TNF, CXCL12, CCL3 and CCL4) (Fig. 6f). We observed significant increases in these innate immune responses in the stromal components (fibroblast, adventitial cell and myofibroblast) of the IC-LOC as well as in the expected innate immune cell types (Fig. 6f), highlighting the role of both the stromal and tissue-resident immune cells in the tissue-level response to infection.
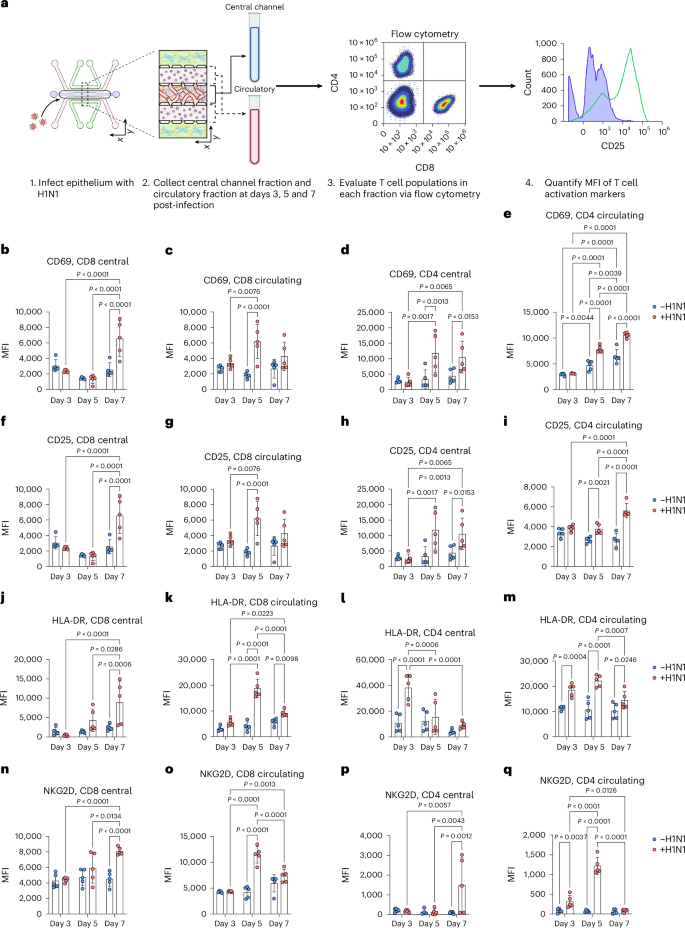
We further validated the activation of CD4 T, CD8 T and NK cells via flow cytometry and observed significant increases in activation profiles in response to severe influenza in the IC-LOC (Extended Data Fig. 9). While we observed modest but significant activation of the T cell subsets at 3 days post-infection, we further investigated whether this activation could be sustained over the course of 7 days, as naive T cell activation is typically triggered within the first 3 days of infection, with differentiation and effector function occurring by day 7 and beyond. To this end, we evaluated the expression levels of activation markers CD25, CD69, HLA-DR and NKG2D in both CD4 and CD8 T cell populations collected from the media channels (denoted as ‘circulatory’) or the degraded central channel (denoted as ‘central’) of both uninfected and infected IC-LOC devices at 3, 5 and 7 days post-infection (Fig. 7a and Supplementary Fig. 18). With this, we found that while activation of the CD8 T cell populations was indeed initiated at 3 days post-infection with significantly elevated levels of CD69 (Fig. 7b,c), expression of CD25 (Fig. 7f,g), HLA-DR (Fig. 7j,k) and NKG2D (Fig. 7n,o) continued to increase at 5 and 7 days post-infection. In addition, we observed that CD4 T cells in the central channel exhibited a nearly 3-fold increase in HLA-DR expression 3 days post-infection (Fig. 7l,m), followed by 2- to 3-fold increases in CD69 (Fig. 7d,e) and CD25 (Fig. 7h,i) by days 5 and 7. While CD4 T cells in the circulating fraction also showed significant increases in CD25, CD69 and HLA-DR, they did not reach the magnitude of those in the central channel, thereby highlighting a unique capability of our IC-LOC system to distinctly evaluate the activation of cells residing within the interstitial (‘central’) regions of the lung device and the more distant ‘circulatory’ fraction.
a, Schematic showing experimental set-up, created using BioRender (https://BioRender.com/f8dciz7). b–e, CD69 expression levels at 3, 5 and 7 days post-infection, as shown by MFI, in central channel CD8 T cells (b), circulating CD8 T cells (c), central channel CD4 T cells (d) and circulating CD4 T cells (e) from uninfected and infected IC-LOC devices. N = 5 independent devices at each timepoint for each condition. f–i, CD25 expression levels at 3, 5 and 7 days post-infection, as shown by MFI, in central channel CD8 T cells (f), circulating CD8 T cells (g), central channel CD4 T cells (h) and circulating CD4 T cells (i) from uninfected and infected IC-LOC devices. N = 5 independent devices at each timepoint for each condition. j–m, HLA-DR expression levels at 3, 5 and 7 days post-infection, as shown by MFI, in central channel CD8 T cells (j), circulating CD8 T cells (k), central channel CD4 T cells (l) and circulating CD4 T cells (m) from uninfected and infected IC-LOC devices. N = 5 independent devices at each timepoint for each condition. n–q, NKG2D expression levels at 3, 5 and 7 days post-infection, as shown by MFI, in central channel CD8 T cells (n), circulating CD8 T cells (o), central channel CD4 T cells (p) and circulating CD4 T cells (q) from uninfected and infected IC-LOC devices. N = 5 independent devices at each timepoint for each condition. All devices use immune cells from the same immune cell donor. Statistical significance determined via ordinary one-way ANOVA followed by Tukey’s multiple comparisons test with P values shown on plots. Data are presented as mean values ± s.d.
In addition to gene expression analysis, we utilized CellChat analysis techniques to evaluate the change in predicted cellular communication and cell–cell interactions in response to severe H1N149. In the healthy state, the primary interactions were between myofibroblasts, IMs, adventitial cells, endothelial cells and epithelial cells (Fig. 6g and Extended Data Fig. 10a). Upon H1N1 infection, interactions with fibroblasts, CD4 T cells, granulocytes and DCs increased dramatically, as can be seen in Fig. 6g, with the top 10% of predicted interactions shown and line thickness corresponding to the number of predicted interactions. Notably, we again observe the significant role of stromal cells, namely fibroblasts, in severe influenza in the IC-LOC.
CXCR4, IL-1β and TNF-α inhibition alters severe influenza
We then used the joint manifold learning capabilities of CellChat to cluster the significant pathways by function and observed six distinct clusters (Extended Data Fig. 10a). We computed the Euclidean distance between control pathways and their 8 h H1N1 counterparts to quantify the functional difference between pathways from uninfected and infected samples, with a larger Euclidean distance indicative of increased dysfunction (Extended Data Fig. 10b,c). Through this analysis, we found that the TNF and IL-1 pathways were implicated in the sequencing data as dysregulated pathways in response to H1N1 (Extended Data Fig. 10c), in agreement with the cytokine secretion data (Fig. 2j). Thus, we chose to evaluate TNF-α and IL-1β inhibitors for their ability to ameliorate the cytokine storm associated with severe influenza. To mimic the typical in vivo timeline of antiviral administration, all therapeutics were administered to the devices 24 hpi. After 24 h of treatment (48 hpi), media was collected from the interstitial and airway layers of the device, and the secreted cytokine landscape was quantified via LEGENDplex multiplexed cytokine analysis. We found that the IL-1β inhibitor, canakinumab (10 µg ml−1), was uniquely able to control the hyperinflammation following infection, significantly reducing cytokine levels compared with untreated devices and, in most cases, returning the levels back to that of the uninfected devices (Fig. 8a,b). While administration of the TNF-α inhibitor, infliximab (10 µg ml−1), had a modest anti-inflammatory effect in the interstitium, it resulted in significant spikes in the airway layer in antiviral response cytokines CXCL10, IFN-λ1 and IFN-λ2/3 and pro-inflammatory IL-1β, further contributing to the hyperinflammatory environment (Fig. 8a,b). We also wondered whether the antiviral oseltamivir (Tamiflu), approved for seasonal flu by United States Food and Drug Administration, could mitigate the associated inflammatory response in severe influenza. Notably, we found that administering Tamiflu (100 µM) to severe influenza IC-LOC devices had little to no beneficial effect on the observed cytokine response, even increasing levels of IL-1β and GM-CSF in the airway (Fig. 8a,b).
a,b, Cytokine release profiles in the interstitium (a) and airway (b) of IC-LOC devices in response to infection and treatment with Tamiflu, IL-1βi (canakinumab) or TNF-αi (infliximab). N = 5 independent devices. c, CellChat predicted L–R interactions comprising the CXCL pathway, with CXCL12–CXCR4 appearing as the key L–R pair. d,e, CXCR4 expression levels in CXCR4+ NK cells (d) and CD8 T cells (e) in uninfected and infected devices, determined from scRNA-seq data. Significance determined via one-way ANOVA followed by Kruskal–Wallis test. f, Flow cytometry quantification of live PBMC cell counts per device in the circulatory fraction of IC-LOC devices in response to H1N1 infection and treatment with CXCR4 inhibitor AMD3100. N = 5 samples for ±H1N1 and N = 3 samples for AMD3100, with each sample comprised of 3 pooled IC-LOC devices. g,h, Activation phenotypes of NK cells (g) and CD8 T cells (h) in response to influenza infection and CXCR4 inhibition with AMD3100. Marker expression plotted as fold change from uninfected devices. N = 5 samples for ±H1N1 and N = 3 samples for AMD3100, with each sample comprised of 3 pooled IC-LOC devices. i, Cytokine release profiles in the interstitium of IC-LOC devices in response to infection and treatment with AMD3100. N = 10 independent devices across at least 2 independent experiments. j,k, Quantified viral levels as determined via particle count from confocal fluorescence microscopy in the airway (j) and interstitium (k) of infected devices left untreated or treated with Tamiflu, IL-1βi (canakinumab), TNF-αi (infliximab) or CXCR4i (AMD3100). N = 18 ROIs from 3 independent devices. All devices use immune cells from the same immune cell donor. Statistical significance determined via ordinary one-way ANOVA unless otherwise specified. Data are presented as mean values ± s.d.
Notably, among the signalling pathways canonically associated with inflammation, it was the CXCL pathway that had the largest predicted dysregulation between the control and 8 h H1N1 samples (Extended Data Fig. 10c). Thus, we investigated the specific signalling occurring in this pathway. When considering the specific ligand–receptor (L–R) interactions, we found that the CXCL12–CXCR4 interaction had the largest relative contribution in the CXCL pathway, coming largely from fibroblast CXCL12 (Fig. 8c and Extended Data Fig. 10d). CXCR4 is known to be involved in leukocyte recruitment and, in epithelial cells, has been shown to regulate mucosal host defence69,70,71. We evaluated CXCL12 expression levels in the interstitium of the IC-LOC via confocal microscopy and found a significant increase in response to severe influenza (Extended Data Fig. 10e). We then probed CXCR4 expression levels across adaptive immune populations and found that in the cells expressing CXCR4, the expression levels in CD8 T and NK cells were significantly upregulated in response to infection, according to scRNA-seq analysis (Fig. 8d,e). Thus, we hypothesized that this axis may play a role in recruiting leukocytes from the media channels into the central channel of infected devices. To evaluate this, we treated devices with a CXCR4 inhibitor, AMD3100 (1 µg ml−1), 1 h before infection. At the endpoint (72 hpi), we collected all circulating immune cells from the media channels and found that while H1N1 infection significantly decreased the numbers of circulatory immune cells in the media channels, indicative of an increase in migration into the central channel as seen in Fig. 4j,k, treatment with AMD3100 restored this number nearly back to uninfected levels (Fig. 8f).
We also evaluated the effect of CXCR4 inhibition via AMD3100 on the activation state of the collected PBMCs through flow cytometric analysis. We found that while H1N1 infection resulted in significant increases in activation markers CD25, CD69, HLA-DR, granzyme B, IFNγ and NKp44 on CD8 T and NK cells, the addition of AMD3100 did not reduce a majority of these markers except HLA-DR and granzyme B in CD8 T cells (Fig. 8g,h). Thus, it appears that while this axis plays a prominent role in immune cell migration, it does not majorly impact the activation state of canonical effectors, CD8 T and NK cells. We further probed the effect of inhibiting this axis on the resultant cytokine storm following severe influenza infection. We observed that inhibition of CXCR4 resulted in a decrease in inflammatory and regulatory cytokines (IL-1β, TNF-α, IL-10 and GM-CSF) and an increase in antiviral-associated cytokines (CXCL10, IFN-λ1, IFN-λ2/3 and IFN-α2) (Fig. 8i). Thus, CXCR4 inhibition could be a potential therapeutic target in the treatment of severe influenza, as it does not hamper the necessary activation of effector immune cells (CD8 T and NK) and increases the antiviral cytokine response while simultaneously decreasing levels of cytokines associated with tissue damage, namely IL-1β, TNF-α and GM-CSF.
To evaluate the potential of each inhibitor to reduce viral load, we then quantified the detectable levels of viral particles in the airway and interstitial regions of the devices. We found that while Tamiflu did not reduce cytokine levels, it significantly reduced the viral levels in both layers of the device (Fig. 8j,k). TNF-α inhibition also significantly reduced viral levels in the interstitium but had no effect in the airway layer of the device. Meanwhile, IL-1β inhibition did not significantly affect viral levels in the interstitium but significantly increased the observed viral level in the airway, compared with untreated devices. Thus, while IL-1β inhibition effectively ameliorates the observed cytokine storm, it may inhibit the immune response too severely and lead to increased viral presence. When we evaluated the viral levels in devices treated with the CXCR4 inhibitor AMD3100, we found that viral levels in the epithelial and interstitial regions of the device were significantly reduced to levels similar to those resulting from Tamiflu treatment (Fig. 8j,k). This is surprising, as CXCR4 does not directly interfere with viral entry or replication as Tamiflu does. We hypothesize that the significant drop in viral levels is instead a consequence of the significantly increased antiviral cytokines secreted in response to AMD3100 treatment and not a result of CXCR4i-induced epithelial cell death, as the treatment with AMD3100 did not significantly impact the epithelial permeability in the IC-LOC (Extended Data Fig. 10f).
Taken together, we demonstrate the unique roles of IL-1β, TNF-α and CXCR4 in response to severe influenza infection. TNF-α appears critical for the regulation of the epithelial cytokine response as its inhibition results in dramatic spikes in inflammatory and antiviral cytokine secretion. Conversely, IL-1β may be a driver of the hyperinflammatory response to severe influenza, as its inhibition results in the amelioration of the cytokine storm both in the interstitium and airway of the IC-LOC. Meanwhile, we find that CXCR4 is critical for the migration of circulatory immune cells into inflamed tissues and its inhibition significantly modulates the cytokine landscape of the inflamed tissue. In fact, CXCR4 inhibition is unique in its ability to decrease the pro-inflammatory cytokine profiles while increasing the antiviral response, in turn decreasing viral levels and arising as a potential therapeutic target.
Source link